Ustekinumab in the Treatment of Inflammatory Bowel Diseases: Evolving Paradigms
Abstract
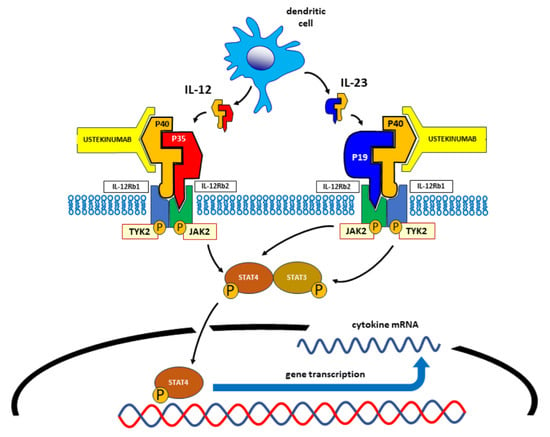
:1. Introduction
2. Materials and Methods
3. Results
3.1. UST in CD
Evidence from Randomized Controlled Trials (RCTs)
3.2. ‘Real-World’ Experience in CD
3.2.1. UST for Bio-Experienced Patients
3.2.2. UST for Bio-Naïve Patients
3.3. UST in UC
3.3.1. Evidence from Randomized Controlled Trials
3.3.2. ‘Real-World’ Experience in UC
3.4. Safety
3.5. UST in Special Situations
3.5.1. UST for Perianal Disease
3.5.2. UST for Postoperative Recurrence
3.5.3. UST for Extraintestinal Manifestations
3.5.4. UST for Pouchitis
3.5.5. UST in Pregnancy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S. Loss of response to anti-tumor necrosis factors: What is the next step? Dig. Dis. 2014, 32, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Hanauer, S.B. Assessing response and loss of response to biological therapies in IBD. Am. J. Gastroenterol. 2011, 106, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Mocci, G.; Lorenzetti, R.; Allegretta, L.; Brandimarte, G.; Cassieri, C.; Colucci, R.; De Medici, A.; Faggiani, R.; Ferronato, A.; et al. Long-term real-life efficacy and safety of infliximab and adalimumab in the treatment of inflammatory bowel diseases outpatients. Eur. J. Gastroenterol. Hepatol. 2021, 33, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Bowman, E.P.; McElwee, J.J.; Smyth, M.J.; Casanova, J.L.; Cooper, A.M.; Cua, D.J. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015, 21, 719–729. [Google Scholar] [CrossRef]
- Simons, N.; Degboe, Y.; Barnetche, T.; Cantagrel, A.; Ruyssen-Witrand, A.; Constantin, A. Biological DMARD efficacy in psoriatic arthritis: A systematic literature review and meta-analysis on articular, enthesitis, dactylitis, skin and functional outcomes. Clin. Exp. Rheumatol. 2020, 38, 508–515. [Google Scholar]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef]
- Parham, C.; Chirica, M.; Timans, J.; Vaisberg, E.; Travis, M.; Cheung, J.; Pflanz, S.; Zhang, R.; Singh, K.P.; Vega, F.; et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002, 168, 5699–5708. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fitz, L.; Ryan, M.; Hewick, R.M.; Clark, S.C.; Chan, S.; Loudon, R.; Sherman, F.; Perussia, B.; Trinchieri, G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989, 170, 827–845. [Google Scholar] [CrossRef]
- Presky, D.H.; Yang, H.; Minetti, L.J.; Chua, A.O.; Nabavi, N.; Wu, C.Y.; Gately, M.K.; Gubler, U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 14002–14007. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ghilardi, N.; Xie, M.H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003, 278, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.M.; Sachs, C.W.; Treacy, G.; Zhou, H.; Pendley, C.E.; Brodmerkel, C.M.; Shankar, G.; Mascelli, M.A. Therapeutic targeting of the IL-12/23 pathways: Generation and characterization of ustekinumab. Nat. Biotechnol. 2011, 29, 615–624. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor. Rev. 2019, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Gasink, C.; Gao, L.L.; Blank, M.A.; Johanns, J.; Guzzo, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Rutgeerts, P.; et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N. Engl. J. Med. 2012, 367, 1519–1528. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Sandborn, W.J.; Feagan, B.G.; Gasink, C.; Jacobstein, D.; Zou, B.; Johanns, J.; Adedokun, O.J.; Sands, B.E.; Rutgeerts, P.; et al. IM-UNITI: Three-year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn’s Disease. J. Crohns Colitis 2020, 14, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Rowbotham, D.S.; Danese, S.; Sandborn, W.J.; Miao, Y.; Zhang, H.; Tikhonov, I.; Panaccione, R.; Hisamatsu, T.; Scherl, E.J.; et al. Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-term Extension. J. Crohns Colitis 2022, 16, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Iborra, M.; Beltran, B.; Fernandez-Clotet, A.; Gutierrez, A.; Antolin, B.; Huguet, J.M.; De Francisco, R.; Merino, O.; Carpio, D.; Garcia-Lopez, S.; et al. Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharmacol. Ther. 2019, 50, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Liefferinckx, C.; Verstockt, B.; Gils, A.; Noman, M.; Van Kemseke, C.; Macken, E.; De Vos, M.; Van Moerkercke, W.; Rahier, J.F.; Bossuyt, P.; et al. Long-term Clinical Effectiveness of Ustekinumab in Patients with Crohn’s Disease Who Failed Biologic Therapies: A National Cohort Study. J. Crohns Colitis 2019, 13, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Biemans, V.B.C.; van der Meulen-de Jong, A.E.; van der Woude, C.J.; Lowenberg, M.; Dijkstra, G.; Oldenburg, B.; de Boer, N.K.H.; van der Marel, S.; Bodelier, A.G.L.; Jansen, J.M.; et al. Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J. Crohns Colitis 2020, 14, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Krisam, J.; Wehling, C.; Kloeters-Plachky, P.; Leopold, Y.; Belling, N.; Gauss, A. Ustekinumab: “Real-world” outcomes and potential predictors of nonresponse in treatment-refractory Crohn’s disease. World J. Gastroenterol. 2019, 25, 4481–4492. [Google Scholar] [CrossRef]
- Eberl, A.; Hallinen, T.; Af Bjorkesten, C.G.; Heikkinen, M.; Hirsi, E.; Kellokumpu, M.; Koskinen, I.; Moilanen, V.; Nielsen, C.; Nuutinen, H.; et al. Ustekinumab for Crohn’s disease: A nationwide real-life cohort study from Finland (FINUSTE). Scand. J. Gastroenterol. 2019, 54, 718–725. [Google Scholar] [CrossRef]
- Bar-Gil Shitrit, A.; Ben-Ya’acov, A.; Siterman, M.; Waterman, M.; Hirsh, A.; Schwartz, D.; Zittan, E.; Adler, Y.; Koslowsky, B.; Avni-Biron, I.; et al. Safety and effectiveness of ustekinumab for induction of remission in patients with Crohn’s disease: A multicenter Israeli study. United Eur. Gastroenterol. J. 2020, 8, 418–424. [Google Scholar] [CrossRef]
- Bennett, A.; Evers Carlini, L.; Duley, C.; Garrett, A.; Annis, K.; Wagnon, J.; Dalal, R.; Scoville, E.; Beaulieu, D.; Schwartz, D.; et al. A Single Center Experience With Long-Term Ustekinumab Use and Reinduction in Patients With Refractory Crohn Disease. Crohns Colitis 360 2020, 2, otaa013. [Google Scholar] [CrossRef]
- Pugliese, D.; Daperno, M.; Fiorino, G.; Savarino, E.; Mosso, E.; Biancone, L.; Testa, A.; Sarpi, L.; Cappello, M.; Bodini, G.; et al. Real-life effectiveness of ustekinumab in inflammatory bowel disease patients with concomitant psoriasis or psoriatic arthritis: An IG-IBD study. Dig. Liver Dis. 2019, 51, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Fedorak, R.N.; Kaplan, G.G.; Dieleman, L.A.; Devlin, S.M.; Stern, N.; Kroeker, K.I.; Seow, C.H.; Leung, Y.; Novak, K.L.; et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: Real world experience from a multicentre cohort. Aliment. Pharmacol. Ther. 2017, 45, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Fries, W.; Viola, A.; Costantino, G.; Muscianisi, M.; Cappello, M.; Guida, L.; Giuffrida, E.; Magnano, A.; Pluchino, D.; et al. Effectiveness of Ustekinumab on Crohn’s Disease Associated Spondyloarthropathy: Real-World Data from the Sicilian Network for Inflammatory Bowel Diseases (SN-IBD). Expert Opin. Biol. Ther. 2020, 20, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Muscianisi, M.; Macaluso, F.S.; Ventimiglia, M.; Cappello, M.; Privitera, A.C.; Magnano, A.; Pluchino, D.; Magri, G.; Ferracane, C.; et al. Ustekinumab in Crohn’s disease: Real-world outcomes from the Sicilian network for inflammatory bowel diseases. JGH Open 2021, 5, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Gravina, A.G.; Cuomo, A.; Mucherino, C.; Sgambato, D.; Facchiano, A.; Granata, L.; Priadko, K.; Pellegrino, R.; de Filippo, F.R.; et al. Efficacy of ustekinumab in the treatment of patients with Crohn’s disease with failure to previous conventional or biologic therapy: A prospective observational real-life study. J. Physiol. Pharmacol. 2021, 72, 5. [Google Scholar] [CrossRef]
- Yokoyama, S.; Asano, T.; Nagano, K.; Tsuchiya, H.; Takagishi, M.; Tsujioka, S.; Miura, N.; Matsumoto, T. Safety and effectiveness of ustekinumab in Crohn’s disease: Interim results of post-marketing surveillance in Japan. J. Gastroenterol. Hepatol. 2021, 36, 3069–3076. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.Y.; Zhang, M.; Wang, W.; Peng, X.; Zhao, J.Z.; Liu, T.; Li, Z.W.; Sun, H.T.; Hu, P.; Zhi, M. Ustekinumab trough concentration affects clinical and endoscopic outcomes in patients with refractory Crohn’s disease: A Chinese real-world study. BMC Gastroenterol. 2021, 21, 380. [Google Scholar] [CrossRef]
- Tursi, A.; Mocci, G.; Cuomo, A.; Allegretta, L.; Aragona, G.; Colucci, R.; Della Valle, N.; Ferronato, A.; Forti, G.; Gaiani, F.; et al. Real-life efficacy and safety of Ustekinumab as second- or third-line therapy in Crohn’s disease: Results from a large Italian cohort study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2099–2108. [Google Scholar] [CrossRef]
- Scribano, M.L.; Aratari, A.; Neri, B.; Bezzio, C.; Balestrieri, P.; Baccolini, V.; Falasco, G.; Camastra, C.; Pantanella, P.; Monterubbianesi, R.; et al. Effectiveness of ustekinumab in patients with refractory Crohn’s disease: A multicentre real-life study in Italy. Therap Adv. Gastroenterol. 2022, 15, 17562848211072412. [Google Scholar] [CrossRef]
- Ylisaukko-Oja, T.; Puttonen, M.; Jokelainen, J.; Koivusalo, M.; Tamminen, K.; Torvinen, S.; Voutilainen, M. Dose-escalation of adalimumab, golimumab or ustekinumab in inflammatory bowel diseases: Characterization and implications in real-life clinical practice. Scand. J. Gastroenterol. 2022, 57, 415–423. [Google Scholar] [CrossRef]
- Parra, R.S.; Chebli, J.M.F.; Queiroz, N.S.F.; Damiao, A.; de Azevedo, M.F.C.; Chebli, L.A.; Bertges, E.R.; Alves Junior, A.J.T.; Ambrogini Junior, O.; da Silva, B.; et al. Long-term effectiveness and safety of ustekinumab in bio-naive and bio-experienced anti-tumor necrosis factor patients with Crohn’s disease: A real-world multicenter Brazilian study. BMC Gastroenterol. 2022, 22, 199. [Google Scholar] [CrossRef] [PubMed]
- Forss, A.; Clements, M.; Myrelid, P.; Strid, H.; Soderman, C.; Wagner, A.; Andersson, D.; Hjelm, F.; Olen, O.; The PROSE SWIBREG Study Group; et al. Ustekinumab Is Associated with Real-World Long-Term Effectiveness and Improved Health-Related Quality of Life in Crohn’s Disease. Dig. Dis. Sci. 2023, 68, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Ihara, Y.; Tominaga, N.; Takedomi, H.; Tsuruoka, N.; Akutagawa, T.; Yukimoto, T.; Kawasaki, K.; Umeno, J.; Torisu, T.; et al. Predictive factors of the clinical efficacy of ustekinumab in patients with refractory Crohn’s disease: Tertiary centers experience in Japan. Int. J. Colorectal Dis. 2023, 38, 57. [Google Scholar] [CrossRef] [PubMed]
- Casas-Deza, D.; Lamuela-Calvo, L.J.; Gomollon, F.; Arbones-Mainar, J.M.; Caballol, B.; Gisbert, J.P.; Rivero, M.; Sanchez-Rodriguez, E.; Arias Garcia, L.; Gutierrez Casbas, A.; et al. Effectiveness and Safety of Ustekinumab in Elderly Patients with Crohn’s Disease: Real World Evidence From the ENEIDA Registry. J. Crohns Colitis 2023, 17, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo Gonzalez, L.; Valdes Delgado, T.; Vazquez Moron, J.M.; Castro Laria, L.; Leo Carnerero, E.; Maldonado Perez, M.B.; Sanchez Capilla, D.; Pallares Manrique, H.; Saez Diaz, A.; Arguelles Arias, F.; et al. Ustekinumab in Crohn’s disease: Real-world outcomes and predictors of response. Rev. Esp. Enferm. Dig. 2022, 114, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Bacaksiz, F.; Ari, D.; Gokbulut, V.; Ozturk, O.; Kayacetin, E. One-year real life data of our patients with moderate-severe Crohn’s disease who underwent ustekinumab therapy. Scott. Med. J. 2021, 66, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Garre, A.; Iborra, M.; Sierra-Ausin, M.; Barreiro-de Acosta, M.; Fernandez-Clotet, A.; de Castro, L.; Bosca-Watts, M.; Casanova, M.J.; Lopez-Garcia, A.; et al. Effectiveness and Safety of Ustekinumab in Ulcerative Colitis: Real-world Evidence from the ENEIDA Registry. J. Crohns Colitis 2021, 15, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Fumery, M.; Filippi, J.; Abitbol, V.; Biron, A.; Laharie, D.; Serrero, M.; Altwegg, R.; Bouhnik, Y.; Peyrin-Biroulet, L.; Gilletta, C.; et al. Effectiveness and safety of ustekinumab maintenance therapy in 103 patients with ulcerative colitis: A GETAID cohort study. Aliment Pharmacol. Ther. 2021, 54, 944–951. [Google Scholar] [CrossRef]
- Amiot, A.; Filippi, J.; Abitbol, V.; Cadiot, G.; Laharie, D.; Serrero, M.; Altwegg, R.; Bouhnik, Y.; Peyrin-Biroulet, L.; Gilletta, C.; et al. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: A GETAID multicentre real-world cohort study. Aliment. Pharmacol. Ther. 2020, 51, 1039–1046. [Google Scholar] [CrossRef]
- Chiappetta, M.F.; Viola, A.; Mastronardi, M.; Turchini, L.; Carparelli, S.; Orlando, A.; Biscaglia, G.; Miranda, A.; Guida, L.; Costantino, G.; et al. One-year effectiveness and safety of ustekinumab in ulcerative colitis: A multicenter real-world study from Italy. Expert. Opin. Biol. Ther. 2021, 21, 1483–1489. [Google Scholar] [CrossRef]
- Hong, S.J.; Krugliak Cleveland, N.; Akiyama, S.; Zullow, S.; Yi, Y.; Shaffer, S.R.; Malter, L.B.; Axelrad, J.E.; Chang, S.; Hudesman, D.P.; et al. Real-World Effectiveness and Safety of Ustekinumab for Ulcerative Colitis From 2 Tertiary IBD Centers in the United States. Crohns Colitis 360 2021, 3, otab002. [Google Scholar] [CrossRef]
- Honap, S.; Al-Hillawi, L.; Baillie, S.; Bancil, A.; Matini, L.; Lau, R.; Kok, K.B.; Patel, K.; Walsh, A.; Irving, P.M.; et al. Ustekinumab for the treatment of moderate to severe ulcerative colitis: A multicentre UK cohort study. Frontline Gastroenterol. 2022, 13, 517–523. [Google Scholar] [CrossRef]
- Parakkal, D.; Johnson, A.; Fenster, M.; Ramos, G.; Zulqarnain, M.; Ullman, T.; Huang, L.; Gutierrez, A.; Bruss, A.; Ungaro, R.; et al. Real-World Effectiveness And Safety Of Ustekinumab In Patients With Ulcerative Colitis: A Multi-Centre Study. J. Crohn’s Colitis 2021, 15, S349–S350. [Google Scholar] [CrossRef]
- Ecker, D.; Fuchssteiner, H.; Gregus, M.; Piringer, P.; Wewalka, F.; Schöfl, R.; Kienbauer, M. Ustekinumab for Ulcerative Colitis A Real-World Experience—Retrospective Data Analysis of the IBD Cohort Ordensklinikum Linz. United Eur. Gastroenterol. J. 2021, 9, 490. [Google Scholar] [CrossRef]
- Alsoud, D.; De Hertogh, G.; Compernolle, G.; Tops, S.; Sabino, J.; Ferrante, M.; Thomas, D.; Vermeire, S.; Verstockt, B. Real-world Endoscopic and Histological Outcomes Are Correlated with Ustekinumab Exposure in Patients with Ulcerative Colitis. J. Crohns Colitis 2022, 16, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Thunberg, J.; Bjorkqvist, O.; Hedin, C.R.H.; Forss, A.; Soderman, C.; Bergemalm, D.; Group, S.S.; Olen, O.; Hjortswang, H.; Strid, H.; et al. Ustekinumab treatment in ulcerative colitis: Real-world data from the Swedish inflammatory bowel disease quality register. United Eur. Gastroenterol. J. 2022, 10, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ochsenkuhn, T.; Tillack, C.; Szokodi, D.; Janelidze, S.; Schnitzler, F. Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis. United Eur. Gastroenterol. J. 2020, 8, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Irving, P.M.; Hoops, T.; Izanec, J.L.; Gao, L.L.; Gasink, C.; Greenspan, A.; Allez, M.; Danese, S.; Hanauer, S.B.; et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: A multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 2022, 399, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Chapuis-Biron, C.; Kirchgesner, J.; Pariente, B.; Bouhnik, Y.; Amiot, A.; Viennot, S.; Serrero, M.; Fumery, M.; Allez, M.; Siproudhis, L.; et al. Ustekinumab for Perianal Crohn’s Disease: The BioLAP Multicenter Study From the GETAID. Am. J. Gastroenterol. 2020, 115, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Godoy Brewer, G.M.; Salem, G.; Afzal, M.A.; Limketkai, B.N.; Haq, Z.; Tajamal, M.; Melia, J.; Lazarev, M.; Selaru, F.M.; Parian, A.M. Ustekinumab is effective for perianal fistulising Crohn’s disease: A real-world experience and systematic review with meta-analysis. BMJ Open Gastroenterol. 2021, 8, e000702. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, H.; Su, T.; Peng, X.; Zhao, J.; Liu, T.; Wang, W.; Hu, P.; Zhi, M.; Zhang, M. Ustekinumab Promotes Radiological Fistula Healing in Perianal Fistulizing Crohn’s Disease: A Retrospective Real-World Analysis. J. Clin. Med. 2023, 12, 939. [Google Scholar] [CrossRef]
- Mañosa, M.; Fernandez-Clotet, A.; Nos, P.; Martin-Arranz, M.D.; Manceñido, N.; Carbajo, A.; Hinojosa, E.; Hernandez-Camba, A.; Muñoz-Perez, R.; Bosca-Watts, M.; et al. Ustekinumab and vedolizumab for the prevention of postoperative recurrence of Crohn’s disease: Results from the ENEIDA registry. Dig. Liver Dis. 2023, 55, 46–52. [Google Scholar] [CrossRef]
- Tursi, A.; Mocci, G.; Picchio, M.; Elisei, W.; Maconi, G. Letter: Ustekinumab for the treatment of post-surgical and refractory Crohn’s disease. Aliment. Pharmacol. Ther. 2021, 53, 859–860. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Grova, M.; Mocciaro, F.; Di Mitri, R.; Privitera, A.C.; Distefano, M.E.; Vitello, A.; Camilleri, S.; Ferracane, C.; Pluchino, D.; et al. Ustekinumab is a promising option for the treatment of postoperative recurrence of Crohn’s disease. J. Gastroenterol. Hepatol. 2023, 38, 1503–1509. [Google Scholar] [CrossRef]
- Guillo, L.; D’Amico, F.; Danese, S.; Peyrin-Biroulet, L. Ustekinumab for Extra-intestinal Manifestations of Inflammatory Bowel Disease: A Systematic Literature Review. J. Crohns Colitis 2021, 15, 1236–1243. [Google Scholar] [CrossRef]
- Ollech, J.E.; Rubin, D.T.; Glick, L.; Weisshof, R.; El Jurdi, K.; Israel, A.; Krugliak Cleveland, N.; Hyman, N.; Sakuraba, A.; Pekow, J.; et al. Ustekinumab Is Effective for the Treatment of Chronic Antibiotic-Refractory Pouchitis. Dig. Dis. Sci. 2019, 64, 3596–3601. [Google Scholar] [CrossRef]
- Rocchi, C.; Soliman, Y.Y.; Massidda, M.; Vadala di Prampero, S.F.; Bulajic, M.; Sorrentino, D. Is Ustekinumab Effective in Refractory Crohn’s Disease of the Pouch and Chronic Pouchitis? A Systematic Review. Dig. Dis. Sci. 2022, 67, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Allez, M.; Karmiris, K.; Louis, E.; Van Assche, G.; Ben-Horin, S.; Klein, A.; Van der Woude, J.; Baert, F.; Eliakim, R.; Katsanos, K.; et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: Definitions, frequency and pharmacological aspects. J. Crohns Colitis 2010, 4, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Chowers, Y.; Sturm, A.; Sans, M.; Papadakis, K.; Gazouli, M.; Harbord, M.; Jahnel, J.; Mantzaris, G.J.; Meier, J.; Mottet, C.; et al. Report of the ECCO workshop on anti-TNF therapy failures in inflammatory bowel diseases: Biological roles and effects of TNF and TNF antagonists. J. Crohns Colitis 2010, 4, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Yung, D.E.; Ma, C.; Pariente, B.; Wlls, P.; Eliakim, R.; Ungar, B.; Ben-Horin, S.; Kopylov, U. Effectiveness and safety of Ustekinumab for Crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig. Liver Dis. 2019, 51, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Maida, M.; Ventimiglia, M.; Cottone, M.; Orlando, A. Effectiveness and safety of Ustekinumab for the treatment of Crohn’s disease in real-life experiences: A meta-analysis of observational studies. Expert. Opin. Biol. Ther. 2020, 20, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Venkata, K.; Zhang, N.; Malik, T.A. Comparative Effectiveness of Ustekinumab Versus Adalimumab in Induction of Clinical Response and Remission in Crohn’s Disease: Experience of a Real-World Cohort at a Tertiary Care Inflammatory Bowel Disease Referral Center. Gastroenterol. Res. 2019, 12, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Alric, H.; Amiot, A.; Kirchgesner, J.; Treton, X.; Allez, M.; Bouhnik, Y.; Beaugerie, L.; Carbonnel, F.; Meyer, A. The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment Pharmacol. Ther. 2020, 51, 948–957. [Google Scholar] [CrossRef]
- Lenti, M.V.; Levison, S.; Eliadou, E.; Willert, R.; Kemp, K.; Carter, A.; Stansfield, C.; Assadsangabi, A.; Singh, S.; Crooks, B.; et al. A real-world, long-term experience on effectiveness and safety of vedolizumab in adult patients with inflammatory bowel disease: The Cross Pennine study. Dig. Liver Dis. 2018, 50, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kim, J.; Luo, J.; Paul, P.; Rudrapatna, V.; Park, S.; Zheng, K.; Syal, G.; Ha, C.; Fleshner, P.; et al. Comparative Safety and Effectiveness of Biologic Therapy for Crohn’s Disease: A CA-IBD Cohort Study. Clin. Gastroenterol. Hepatol. 2023, 21, 2359–2369.e2355. [Google Scholar] [CrossRef] [PubMed]
- Onali, S.; Pugliese, D.; Caprioli, F.A.; Orlando, A.; Biancone, L.; Nardone, O.M.; Imperatore, N.; Fiorino, G.; Cappello, M.; Viola, A.; et al. An Objective Comparison of Vedolizumab and Ustekinumab Effectiveness in Crohn’s Disease Patients’ Failure to TNF-Alpha Inhibitors. Am. J. Gastroenterol. 2022, 117, 1279–1287. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Adimadhyam, S.; Hou, L.; Wolfe, A.E.; Smith, S.; Simon, A.L.; Moyneur, E.; Reynolds, J.S.; Toh, S.; Dobes, A.; et al. Real-World Evidence Comparing Vedolizumab and Ustekinumab in Antitumor Necrosis Factor-Experienced Patients With Crohn’s Disease. Am. J. Gastroenterol. 2023, 118, 674–684. [Google Scholar] [CrossRef]
- Johnson, A.M.; Barsky, M.; Ahmed, W.; Zullow, S.; Galati, J.; Jairath, V.; Narula, N.; Peerani, F.; Click, B.H.; Coburn, E.S.; et al. The Real-World Effectiveness and Safety of Ustekinumab in the Treatment of Crohn’s Disease: Results From the SUCCESS Consortium. Am. J. Gastroenterol. 2023, 118, 317–328. [Google Scholar] [CrossRef]
- Monin, L.; Dubois, S.; Reenaers, C.; Van Kemseke, C.; Latour, P.; Van Daele, D.; Vieujean, S.; Seidel, L.; Louis, E. Ustekinumab in bio-naive and bio-failure Crohn’s disease patients: Results from a << real-life >> monocentric cohort. Dig. Liver Dis. 2021, 53, 72–78. [Google Scholar] [CrossRef]
- Valdés Delgado, T.; Olmedo Martín, R.; Iborra, M.; Herrera de Guisé, C.; Fuentes-Valenzuela, E.; Melcarne, L.; Martín-Rodríguez, M.; Kolle Casso, L.; De Castro Parga, L.; Ponferrada Díaz, A.; et al. Effectiveness and safety of ustekinumab in bio-naive Crohn’s disease patients: A multicentre observational retrospective study. Therap Adv. Gastroenterol. 2023, 16, 17562848231153560. [Google Scholar] [CrossRef]
- Sedano, R.; Guizzetti, L.; McDonald, C.; Beaton, M.; Chande, N.; Gregor, J.; Sey, M.; Wilson, A.; Jairath, V. Clinical, Endoscopic, and Radiological Effectiveness of Ustekinumab in Bio-naive Versus Bio-experienced Patients With Crohn’s Disease: Real-world Experience From a Large Canadian Center. Inflamm. Bowel Dis. 2023, 29, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Rivière, P.; Kanters, C.; Pellet, G.; Ni, A.; Hupe, M.; Aboulhamid, N.; Poullenot, F.; Bitton, A.; Zerbib, F.; Lakatos, P.L.; et al. Comparative Effectiveness of Ustekinumab and Anti-TNF Agent as First-Line Biological Therapy in Luminal Crohn’s Disease: A Retrospective Study From 2 Referral Centers. Inflamm. Bowel Dis. 2023, 29, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Zhdanava, M.; Ding, Z.; Manceur, A.M.; Muser, E.; Lefebvre, P.; Holiday, C.; Lafeuille, M.H.; Pilon, D. Treatment persistence among bio-naive patients with Crohn’s disease initiated on ustekinumab or adalimumab. Curr. Med. Res. Opin. 2023, 39, 533–543. [Google Scholar] [CrossRef]
- Moens, A.; Alsoud, D.; Verstockt, B.; Sabino, J.; Ferrante, M.; Vermeire, S. Adalimumab versus ustekinumab as first-line biological in moderate-to-severe Crohn’s disease: Real-life cohort from a tertiary referral center. Eur. J. Gastroenterol. Hepatol. 2022, 34, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Afif, W.; Arasaradnam, R.P.; Abreu, M.T.; Danese, S.; Sandborn, W.J.; Miao, Y.; Zhang, H.; Panaccione, R.; Hisamatsu, T.; Scherl, E.J.; et al. Efficacy and Safety of Ustekinumab for Ulcerative Colitis Through 4 Years: Final Results of the UNIFI Long-term Maintenance Study. Am. J. Gastroenterol. 2023, 10, 14309. [Google Scholar] [CrossRef] [PubMed]
- Iborra, M.; Ferreiro-Iglesias, R.; Maria Dolores, M.A.; Mesonero Gismero, F.; Minguez, A.; Porto-Silva, S.; Garcia-Ramirez, L.; Garcia de la Filia, I.; Aguas, M.; Nieto-Garcia, L.; et al. Real-world long-term effectiveness of ustekinumab in ulcerative colitis: Results from a spanish open-label cohort. Scand. J. Gastroenterol. 2023, 59, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Parody-Rua, E.; Chaparro, M. Efficacy, Effectiveness, and Safety of Ustekinumab for the Treatment of Ulcerative Colitis: A Systematic Review. Inflamm. Bowel Dis. 2023, 30, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Maida, M.; Grova, M.; Crispino, F.; Teresi, G.; Orlando, A.; Orlando, A. Head-to-head comparison of biological drugs for inflammatory bowel disease: From randomized controlled trials to real-world experience. Ther. Adv. Gastroenter 2021, 14, 17562848211010668. [Google Scholar] [CrossRef]
- Taxonera, C.; Olivares, D.; Lopez-Garcia, O.N.; Alba, C. Meta-analysis: Real-world effectiveness and safety of ustekinumab in patients with ulcerative colitis. Aliment Pharmacol. Ther. 2023, 57, 610–619. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Heron, V.; Restellini, S.; Bessissow, T. Comparative Efficacy of Biologic Therapies for Inducing Response and Remission in Fistulizing Crohn’s Disease: Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Inflamm. Bowel Dis. 2023, 29, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Gionchetti, P.; Dignass, A.; Danese, S.; Magro Dias, F.J.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.J.; Sebastian, S.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis 2017, 11, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Van Assche, G.; Gomez-Ulloa, D.; Garcia-Alvarez, L.; Lara, N.; Black, C.M.; Kachroo, S. Systematic Review of Tumor Necrosis Factor Antagonists in Extraintestinal Manifestations in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 25–36.e27. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Mocci, G.; Maconi, G. Effect of Ustekinumab on Extraintestinal Diseases in Refractory Crohn’s Disease. J. Crohns Colitis 2021, 15, 1399–1400. [Google Scholar] [CrossRef] [PubMed]
- Livne-Margolin, M.; Ling, D.; Attia-Konyo, S.; Abitbol, C.M.; Haj-Natour, O.; Ungar, B.; Ben-Horin, S.; Kopylov, U. Ustekinumab and vedolizumab for extraintestinal manifestations in inflammatory bowel disease—A retrospective study. Dig. Liver Dis. 2023, 55, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.; Pugliese, D.; Onali, S.; Petito, V.; Scaldaferri, F.; Gasbarrini, A.; Danese, S.; Armuzzi, A. Combination therapy in inflammatory bowel disease—From traditional immunosuppressors towards the new paradigm of dual targeted therapy. Autoimmun. Rev. 2021, 20, 102832. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.D.; Wahedna, N.A.; Aldulaimi, D.; Hawker, P. Emerging role of dual biologic therapy for the treatment of inflammatory bowel disease. World J. Clin. Cases 2023, 11, 2621–2630. [Google Scholar] [CrossRef]
- Berinstein, E.M.; Sheehan, J.L.; Jacob, J.; Steiner, C.A.; Stidham, R.W.; Shannon, C.; Bishu, S.; Levine, J.; Cohen-Mekelburg, S.A.; Waljee, A.K.; et al. Efficacy and Safety of Dual Targeted Therapy for Partially or Non-responsive Inflammatory Bowel Disease: A Systematic Review of the Literature. Dig. Dis. Sci. 2023, 68, 2604–2623. [Google Scholar] [CrossRef]
- Targownik, L.E.; Singh, H.; Nugent, Z.; Bernstein, C.N. The epidemiology of colectomy in ulcerative colitis: Results from a population-based cohort. Am. J. Gastroenterol. 2012, 107, 1228–1235. [Google Scholar] [CrossRef]
- Solberg, I.C.; Lygren, I.; Jahnsen, J.; Aadland, E.; Hoie, O.; Cvancarova, M.; Bernklev, T.; Henriksen, M.; Sauar, J.; Vatn, M.H.; et al. Clinical course during the first 10 years of ulcerative colitis: Results from a population-based inception cohort (IBSEN Study). Scand. J. Gastroenterol. 2009, 44, 431–440. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Shen, B. Pouchitis: What every gastroenterologist needs to know. Clin. Gastroenterol. Hepatol. 2013, 11, 1538–1549. [Google Scholar] [CrossRef]
- Ferrante, M.; Declerck, S.; De Hertogh, G.; Van Assche, G.; Geboes, K.; Rutgeerts, P.; Penninckx, F.; Vermeire, S.; D’Hoore, A. Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.L.; Shen, B.; Schwartz, D.A. Management of Pouchitis and Other Common Complications of the Pouch. Inflamm. Bowel Dis. 2018, 24, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Bar, F.; Kuhbacher, T.; Dietrich, N.A.; Krause, T.; Stallmach, A.; Teich, N.; Schreiber, S.; Walldorf, J.; Schmelz, R.; Buning, C.; et al. Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment. Pharmacol. Ther. 2018, 47, 581–587. [Google Scholar] [CrossRef]
- Huguet, M.; Pereira, B.; Goutte, M.; Goutorbe, F.; Dubois, A.; Bommelaer, G.; Buisson, A. Systematic Review With Meta-Analysis: Anti-TNF Therapy in Refractory Pouchitis and Crohn’s Disease-Like Complications of the Pouch After Ileal Pouch-Anal Anastomosis Following Colectomy for Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 261–268. [Google Scholar] [CrossRef]
- Godoy-Brewer, G.; Salem, G.; Limketkai, B.; Selaru, F.M.; Grossen, A.; Policarpo, T.; Haq, Z.; Parian, A.M. Use of Biologics for the Treatment of Inflammatory Conditions of the Pouch: A Systematic Review. J. Clin. Gastroenterol. 2024, 58, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.S.; Gupta, S.; Goodrick, H.; Mitri, J.; Allegretti, J.R. Outcomes of Standard and Intensified Dosing of Ustekinumab for Chronic Pouch Disorders. Inflamm. Bowel Dis. 2022, 28, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Fedorak, R.N.; Scherl, E.; Fleisher, M.R.; Katz, S.; Johanns, J.; Blank, M.; Rutgeerts, P.; Ustekinumab Crohn’s Disease Study, G. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008, 135, 1130–1141. [Google Scholar] [CrossRef]
- Toedter, G.P.; Blank, M.; Lang, Y.; Chen, D.; Sandborn, W.J.; de Villiers, W.J. Relationship of C-reactive protein with clinical response after therapy with ustekinumab in Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 2768–2773. [Google Scholar] [CrossRef]
- Lozano, N.A.; Lozano, A.; Marini, V.; Saranz, R.J.; Blumberg, R.S.; Baker, K.; Agresta, M.F.; Ponzio, M.F. Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am. J. Reprod. Immunol. 2018, 80, e12972. [Google Scholar] [CrossRef]
- Beltagy, A.; Aghamajidi, A.; Trespidi, L.; Ossola, W.; Meroni, P.L. Biologics During Pregnancy and Breastfeeding Among Women With Rheumatic Diseases: Safety Clinical Evidence on the Road. Front. Pharmacol. 2021, 12, 621247. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Safety of New Biologics (Vedolizumab and Ustekinumab) and Small Molecules (Tofacitinib) During Pregnancy: A Review. Drugs 2020, 80, 1085–1100. [Google Scholar] [CrossRef]
- Matro, R.; Martin, C.F.; Wolf, D.; Shah, S.A.; Mahadevan, U. Exposure Concentrations of Infants Breastfed by Women Receiving Biologic Therapies for Inflammatory Bowel Diseases and Effects of Breastfeeding on Infections and Development. Gastroenterology 2018, 155, 696–704. [Google Scholar] [CrossRef]
- Yeung, J.; Gooderham, M.J.; Grewal, P.; Hong, C.H.; Lansang, P.; Papp, K.A.; Poulin, Y.; Turchin, I.; Vender, R. Management of Plaque Psoriasis With Biologic Therapies in Women of Child-Bearing Potential Consensus Paper. J. Cutan. Med. Surg. 2020, 24, 3S–14S. [Google Scholar] [CrossRef] [PubMed]
- Klenske, E.; Osaba, L.; Nagore, D.; Rath, T.; Neurath, M.F.; Atreya, R. Drug Levels in the Maternal Serum, Cord Blood and Breast Milk of a Ustekinumab-Treated Patient with Crohn’s Disease. J. Crohns Colitis 2019, 13, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Gorodensky, J.H.; Bernatsky, S.; Afif, W.; Filion, K.B.; Vinet, E. Ustekinumab Safety in Pregnancy: A Comprehensive Review. Arthritis Care Res. 2023, 75, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Wils, P.; Seksik, P.; Stefanescu, C.; Nancey, S.; Allez, M.; Pineton de Chambrun, G.; Altwegg, R.; Gilletta, C.; Vuitton, L.; Viennot, S.; et al. Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: A multicentre cohort study. Aliment. Pharmacol. Ther. 2021, 53, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Gutiérrez, A.; Calviño-Suárez, C.; Huguet, J.M.; Calvo, M.; Aguas, M.; Camargo Camero, R.; de Jorge Turrión, M.A.; Hervías Cruz, D.; López Serrano, P.; et al. Safety of ustekinumab in pregnant patients with inflammatory bowel disease and in their offspring: Results from the DUMBO registry of GETECCU. J. Crohn’s Colitis 2021, 16, i491–i493. [Google Scholar] [CrossRef]
- Chugh, R.; Long, M.D.; Jiang, Y.; Weaver, K.N.; Beaulieu, D.B.; Scherl, E.J.; Mahadevan, U. Maternal and Neonatal Outcomes in Vedolizumab and Ustekinumab Exposed Pregnancies: Results from the PIANO registry. Am. J. Gastroenterol. 2023, 10, 14309. [Google Scholar] [CrossRef]
- Abraham, B.P.; Ott, E.; Busse, C.; Murphy, C.; Miller, L.; Baumgart, D.C.; Scherl, E.; Gasink, C. Ustekinumab Exposure in Pregnant Women From Inflammatory Bowel Disease Clinical Trials: Pregnancy Outcomes Through Up To 5 Years in Crohn’s Disease and 2 Years in Ulcerative Colitis. Crohns Colitis 360 2022, 4, otac025. [Google Scholar] [CrossRef] [PubMed]
- Echeverria-Garcia, B.; Nuno-Gonzalez, A.; Dauden, E.; Vanaclocha, F.; Torrado, R.; Belinchon, I.; Perez-Zafrilla, B.; Grupo de estudio, B. A Case Series of Patients With Psoriasis Exposed to Biologic Therapy During Pregnancy: The BIOBADADERM Register and a Review of the Literature. Actas Dermosifiliogr. 2017, 108, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Dernoncourt, A.; Liabeuf, S.; Bennis, Y.; Masmoudi, K.; Bodeau, S.; Laville, S.; Hurtel-Lemaire, A.S.; Gras-Champel, V.; Batteux, B. Fetal and Neonatal Adverse Drug Reactions Associated with Biologics Taken During Pregnancy by Women with Autoimmune Diseases: Insights from an Analysis of the World Health Organization Pharmacovigilance Database (VigiBase((R))). BioDrugs 2023, 37, 73–87. [Google Scholar] [CrossRef]
- Ghalandari, N.; Crijns, H.; Bergman, J.E.H.; Dolhain, R.; van Puijenbroek, E.P.; Hazes, J.M.W. Reported congenital malformations after exposure to non-tumour necrosis factor inhibitor biologics: A retrospective comparative study in EudraVigilance. Br. J. Clin. Pharmacol. 2022, 88, 5378–5388. [Google Scholar] [CrossRef]
- Mahadevan, U.; Robinson, C.; Bernasko, N.; Boland, B.; Chambers, C.; Dubinsky, M.; Friedman, S.; Kane, S.; Manthey, J.; Sauberan, J.; et al. Inflammatory Bowel Disease in Pregnancy Clinical Care Pathway: A Report From the American Gastroenterological Association IBD Parenthood Project Working Group. Am. J. Obstet. Gynecol. 2019, 220, 308–323. [Google Scholar] [CrossRef]
- Mitrova, K.; Pipek, B.; Bortlik, M.; Bouchner, L.; Brezina, J.; Douda, T.; Drasar, T.; Klvana, P.; Kohout, P.; Leksa, V.; et al. Safety of Ustekinumab and Vedolizumab During Pregnancy-Pregnancy, Neonatal, and Infant Outcome: A Prospective Multicentre Study. J. Crohns Colitis 2022, 16, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Chaparro, M.; Julsgaard, M.; Katsanos, K.; Zelinkova, Z.; Agrawal, M.; Ardizzone, S.; Campmans-Kuijpers, M.; Dragoni, G.; Ferrante, M.; et al. European Crohn’s and Colitis Guidelines on Sexuality, Fertility, Pregnancy, and Lactation. J. Crohns Colitis 2023, 17, 1–27. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocci, G.; Tursi, A.; Onidi, F.M.; Usai-Satta, P.; Pes, G.M.; Dore, M.P. Ustekinumab in the Treatment of Inflammatory Bowel Diseases: Evolving Paradigms. J. Clin. Med. 2024, 13, 1519. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13051519
Mocci G, Tursi A, Onidi FM, Usai-Satta P, Pes GM, Dore MP. Ustekinumab in the Treatment of Inflammatory Bowel Diseases: Evolving Paradigms. Journal of Clinical Medicine. 2024; 13(5):1519. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13051519
Chicago/Turabian StyleMocci, Giammarco, Antonio Tursi, Francesca Maria Onidi, Paolo Usai-Satta, Giovanni Mario Pes, and Maria Pina Dore. 2024. "Ustekinumab in the Treatment of Inflammatory Bowel Diseases: Evolving Paradigms" Journal of Clinical Medicine 13, no. 5: 1519. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13051519