Association between Ustekinumab Trough Levels, Serum IL-22, and Oncostatin M Levels and Clinical and Biochemical Outcomes in Patients with Crohn’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population and Study Design
2.2. Definition and Outcomes
2.3. Measurement of Faecal Calprotectin Levels
2.4. Measurement of CRP
2.5. Measurement of Serum UST Trough Levels
2.6. Cytokine Analysis
2.7. Statistical Analysis
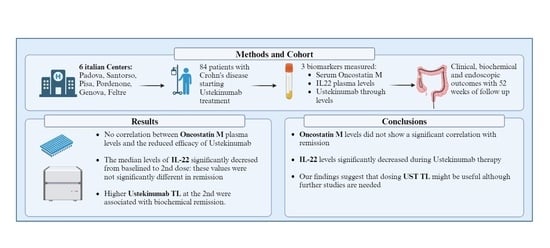
3. Results
3.1. Baseline Characteristics
3.2. Clinical, Biochemical, and Endoscopic Outcomes
3.2.1. Clinical Outcomes
3.2.2. Biochemical Outcomes
3.2.3. Endoscopic Outcomes
3.3. Optimisation of Ustekinumab Dosing
3.4. Safety Profile and Drug Discontinuation
3.5. Ustekinumab Trough Levels
3.6. Oncostatin M Plasma Values
3.7. IL-22 Plasma Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.-P. Long-Term Evolution of Disease Behavior of Crohn’s Disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Loftus, E.V.; Colombel, J.-F.; Sandborn, W.J. The Natural History of Adult Crohn’s Disease in Population-Based Cohorts. Am. J. Gastroenterol. 2010, 105, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, D.; Privitera, G.; Crispino, F.; Mezzina, N.; Castiglione, F.; Fiorino, G.; Laterza, L.; Viola, A.; Bertani, L.; Caprioli, F.; et al. Effectiveness and Safety of Vedolizumab in a Matched Cohort of Elderly and Nonelderly Patients with Inflammatory Bowel Disease: The IG-IBD LIVE Study. Aliment. Pharmacol. Ther. 2022, 56, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Zingone, F.; Barberio, B.; Compostella, F.; Girardin, G.; D’Incà, R.; Marinelli, C.; Marsilio, I.; Lorenzon, G.; Savarino, E.V. Good Efficacy and Safety of Vedolizumab in Crohn’s Disease and Ulcerative Colitis in a Real-World Scenario. Ther. Adv. Gastroenterol. 2020, 13, 1756284820936536. [Google Scholar] [CrossRef]
- Barberio, B.; Zingone, F.; D’Incà, R.; Rovigo, L.; Bertani, L.; Bodini, G.; Ghisa, M.; Gubbiotti, A.; Massimi, D.; Lorenzon, G.; et al. Infliximab Originator, Infliximab Biosimilar, and Adalimumab Are More Effective in Crohn’s Disease than Ulcerative Colitis: A Real-Life Cohort Study. Clin. Transl. Gastroenterol. 2020, 11, e00177. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Pallone, F.; Monteleone, G. Interleukin-12 and Th1 Immune Response in Crohn’s Disease: Pathogenetic Relevance and Therapeutic Inplication. World J. Gastroenterol. 2006, 12, 5606–5610. [Google Scholar] [CrossRef]
- Neurath, M.F. IL-23: A Master Regulator in Crohn Disease. Nat. Med. 2007, 13, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Kubesch, A.; Rueter, L.; Farrag, K.; Krause, T.; Stienecker, K.; Hausmann, J.; Filmann, N.; Dignass, A.; Stein, J.; Blumenstein, I. Short and Long-Term Effectiveness of Ustekinumab in Patients with Crohn’s Disease: Real-World Data from a German IBD Cohort. J. Clin. Med. 2019, 8, 2140. [Google Scholar] [CrossRef]
- Iborra, M.; Beltrán, B.; Fernández-Clotet, A.; Gutiérrez, A.; Antolín, B.; Huguet, J.M.; De Francisco, R.; Merino, O.; Carpio, D.; García-López, S.; et al. Real-World Short-Term Effectiveness of Ustekinumab in 305 Patients with Crohn’s Disease: Results from the ENEIDA Registry. Aliment. Pharmacol. Ther. 2019, 50, 278–288. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Forss, A.; Clements, M.; Myrelid, P.; Strid, H.; Söderman, C.; Wagner, A.; Andersson, D.; Hjelm, F.; Olén, O.; Halfvarson, J.; et al. Ustekinumab Is Associated with Real-World Long-Term Effectiveness and Improved Health-Related Quality of Life in Crohn’s Disease. Dig. Dis. Sci. 2023, 68, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Baston-Rey, I.; Fernández-Salgado, E.; González García, J.; Ramos, L.; Diz-Lois Palomares, M.T.; Argüelles-Arias, F.; Iglesias Flores, E.; Cabello, M.; Rubio Iturria, S.; et al. Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn’s Disease Patients: The SUSTAIN Study. Inflamm. Bowel Dis. 2022, 28, 1725–1736. [Google Scholar] [CrossRef]
- Pugliese, D.; Daperno, M.; Fiorino, G.; Savarino, E.; Mosso, E.; Biancone, L.; Testa, A.; Sarpi, L.; Cappello, M.; Bodini, G.; et al. Real-Life Effectiveness of Ustekinumab in Inflammatory Bowel Disease Patients with Concomitant Psoriasis or Psoriatic Arthritis: An IG-IBD Study. Dig. Liver Dis. 2019, 51, 972–977. [Google Scholar] [CrossRef]
- Ha, C.; Ullman, T.A.; Siegel, C.A.; Kornbluth, A. Patients Enrolled in Randomized Controlled Trials Do Not Represent the Inflammatory Bowel Disease Patient Population. Clin. Gastroenterol. Hepatol. 2012, 10, 1002–1007. [Google Scholar] [CrossRef]
- Wils, P.; Bouhnik, Y.; Michetti, P.; Flourie, B.; Brixi, H.; Bourrier, A.; Allez, M.; Duclos, B.; Serrero, M.; Buisson, A.; et al. Long-Term Efficacy and Safety of Ustekinumab in 122 Refractory Crohn’s Disease Patients: A Multicentre Experience. Aliment. Pharmacol. Ther. 2018, 47, 588–595. [Google Scholar] [CrossRef]
- Burgevin, A.; Caron, B.; Sasson, A.; Luc, A.; Netter, P.; Baumann, C.; Ananthakrishnan, A.N.; Peyrin-Biroulet, L. Comparative Safety of Ustekinumab and Vedolizumab in Older Patients with Inflammatory Bowel Disease: A Bicentric Cohort Study. J. Clin. Med. 2022, 11, 6967. [Google Scholar] [CrossRef]
- Casas-Deza, D.; Lamuela-Calvo, L.J.; Gomollón, F.; Arbonés-Mainar, J.M.; Caballol, B.; Gisbert, J.P.; Rivero, M.; Sánchez-Rodríguez, E.; Arias García, L.; Gutiérrez Casbas, A.; et al. Effectiveness and Safety of Ustekinumab in Elderly Patients with Crohn’s Disease: Real World Evidence from the ENEIDA Registry. J. Crohn’s Colitis 2023, 17, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Fedorak, R.N.; Kaplan, G.G.; Dieleman, L.A.; Devlin, S.M.; Stern, N.; Kroeker, K.I.; Seow, C.H.; Leung, Y.; Novak, K.L.; et al. Clinical, Endoscopic and Radiographic Outcomes with Ustekinumab in Medically-Refractory Crohn’s Disease: Real World Experience from a Multicentre Cohort. Aliment. Pharmacol. Ther. 2017, 45, 1232–1243. [Google Scholar] [CrossRef]
- Kopylov, U.; Afif, W.; Cohen, A.; Bitton, A.; Wild, G.; Bessissow, T.; Wyse, J.; Al-Taweel, T.; Szilagyi, A.; Seidman, E. Subcutaneous Ustekinumab for the Treatment of Anti-TNF Resistant Crohn’s Disease—The McGill Experience✩. J. Crohn’s Colitis 2014, 8, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Taxonera, C.; Olivares, D.; López-García, O.N.; Alba, C. Meta-Analysis: Real-World Effectiveness and Safety of Ustekinumab in Patients with Ulcerative Colitis. Aliment. Pharmacol. Ther. 2023, 57, 610–619. [Google Scholar] [CrossRef]
- Plevris, N.; Fulforth, J.; Siakavellas, S.; Robertson, A.; Hall, R.; Tyler, A.; Jenkinson, P.W.; Campbell, I.; Chuah, C.S.; Kane, C.; et al. Real-World Effectiveness and Safety of Ustekinumab for the Treatment of Crohn’s Disease: The Scottish Ustekinumab Cohort. J. Gastroenterol. Hepatol. 2021, 36, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Gracie, D.J.; Black, C.J.; Ford, A.C. Efficacy of Biological Therapies and Small Molecules in Induction and Maintenance of Remission in Luminal Crohn’s Disease: Systematic Review and Network Meta-Analysis. Gut 2023, 72, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Aladraj, H.; Abdulla, M.; Guraya, S.Y.; Guraya, S.S. Health-Related Quality of Life of Patients Treated with Biological Agents and New Small-Molecule Drugs for Moderate to Severe Crohn’s Disease: A Systematic Review. J. Clin. Med. 2022, 11, 3743. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Parkes, M.; Lee, J.C. How Do We Predict a Patient’s Disease Course and Whether They Will Respond to Specific Treatments? Gastroenterology 2022, 162, 1383–1395. [Google Scholar] [CrossRef]
- Raine, T.; Danese, S. Breaking through the Therapeutic Ceiling: What Will It Take? Gastroenterology 2022, 162, 1507–1511. [Google Scholar] [CrossRef]
- Bertani, L.; Tricò, D.; Pugliese, D.; Privitera, G.; Linsalata, G.; Zanzi, F.; Gloria Mumolo, M.; Barberio, B.; Monzani, F.; Marchi, S.; et al. Serum Triiodothyronine-to-Thyroxine (T3/T4) Ratio Predicts Therapeutic Outcome to Biological Therapies in Elderly IBD Patients. Aliment. Pharmacol. Ther. 2021, 53, 273–280. [Google Scholar] [CrossRef]
- Fiocchi, C.; Dragoni, G.; Iliopoulos, D.; Katsanos, K.; Ramirez, V.H.; Suzuki, K.; Scientific Workshop Steering Committee. Results of the Seventh Scientific Workshop of ECCO: Precision Medicine in IBD-What, Why, and How. J. Crohn’s Colitis 2021, 15, 1410–1430. [Google Scholar] [CrossRef]
- Bertani, L.; Barberio, B.; Fornili, M.; Antonioli, L.; Zanzi, F.; Casadei, C.; Benvenuti, L.; Facchin, S.; D’Antongiovanni, V.; Lorenzon, G.; et al. Serum Oncostatin M Predicts Mucosal Healing in Patients with Inflammatory Bowel Diseases Treated with Anti-TNF, but Not Vedolizumab. Dig. Liver Dis. 2022, 54, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Vande Casteele, N.; Herfarth, H.; Katz, J.; Falck-Ytter, Y.; Singh, S. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 835–857.e6. [Google Scholar] [CrossRef]
- Facchin, S.; Buda, A.; Cardin, R.; Agbariah, N.; Zingone, F.; De Bona, M.; Zaetta, D.; Bertani, L.; Ghisa, M.; Barberio, B.; et al. Rapid Point-of-Care Anti-Infliximab Antibodies Detection in Clinical Practice: Comparison with ELISA and Potential for Improving Therapeutic Drug Monitoring in IBD Patients. Ther. Adv. Gastroenterol. 2021, 14, 1756284821999902. [Google Scholar] [CrossRef]
- Papamichael, K.; Afif, W.; Drobne, D.; Dubinsky, M.C.; Ferrante, M.; Irving, P.M.; Kamperidis, N.; Kobayashi, T.; Kotze, P.G.; Lambert, J.; et al. Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease: Unmet Needs and Future Perspectives. Lancet Gastroenterol. Hepatol. 2022, 7, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Schreiber, S.; Sandborn, W.J.; Dubois, C.; Rutgeerts, P. Correlation between the Crohn’s Disease Activity and Harvey-Bradshaw Indices in Assessing Crohn’s Disease Severity. Clin. Gastroenterol. Hepatol. 2010, 8, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.B.; Lochs, H.; Löfberg, R.; Modigliani, R.; Present, D.H.; Rutgeerts, P.; Schölmerich, J.; Stange, E.F.; et al. A Review of Activity Indices and Efficacy Endpoints for Clinical Trials of Medical Therapy in Adults with Crohn’s Disease. Gastroenterology 2002, 122, 512–530. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A Simple Index of Crohn’s-Disease Activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Zeino, Z.; Tomkins, C.; Cheung, M.; Nwokolo, C.; Smith, S.; Harmston, C.; Arasaradnam, R.P. Utility of Faecal Calprotectin in Inflammatory Bowel Disease (IBD): What Cut-Offs Should We Apply? Frontline Gastroenterol. 2015, 6, 14–19. [Google Scholar] [CrossRef]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and Validation of a New, Simplified Endoscopic Activity Score for Crohn’s Disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Noor, N.M.; Verstockt, B.; Parkes, M.; Lee, J.C. Personalised Medicine in Crohn’s Disease. Lancet Gastroenterol. Hepatol. 2020, 5, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Noor, N.M.; Marigorta, U.M.; Pavlidis, P.; Deepak, P.; Ungaro, R.C.; Scientific Workshop Steering Committee. Results of the Seventh Scientific Workshop of ECCO: Precision Medicine in IBD—Disease Outcome and Response to Therapy. J. Crohn’s Colitis 2021, 15, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Rubín de Célix, C.; Chaparro, M.; Gisbert, J.P. Real-World Evidence of the Effectiveness and Safety of Ustekinumab for the Treatment of Crohn’s Disease: Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Med. 2022, 11, 4202. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef] [PubMed]
- Honap, S.; Meade, S.; Ibraheim, H.; Irving, P.M.; Jones, M.P.; Samaan, M.A. Effectiveness and Safety of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci 2022, 67, 1018–1035. [Google Scholar] [CrossRef] [PubMed]
- Rutz, S.; Eidenschenk, C.; Ouyang, W. IL-22, Not Simply a Th17 Cytokine. Immunol. Rev. 2013, 252, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Yi, T.; Lu, T.T.; Ghilardi, N. The Role of IL-22 in Intestinal Health and Disease. J. Exp. Med. 2020, 217, e20192195. [Google Scholar] [CrossRef]
- Laurence, A.; O’Shea, J.J.; Watford, W.T. Interleukin-22: A Sheep in Wolf’s Clothing. Nat. Med. 2008, 14, 247–249. [Google Scholar] [CrossRef]
- Seiderer, J.; Brand, S. IL-22: A Two-Headed Cytokine in IBD? Inflamm. Bowel Dis. 2009, 15, 473–474. [Google Scholar] [CrossRef]
- Eken, A.; Singh, A.K.; Treuting, P.M.; Oukka, M. IL-23R+ Innate Lymphoid Cells Induce Colitis via Interleukin-22-Dependent Mechanism. Mucosal Immunol. 2014, 7, 143–154. [Google Scholar] [CrossRef]
- Kamanaka, M.; Huber, S.; Zenewicz, L.A.; Gagliani, N.; Rathinam, C.; O’Connor, W., Jr.; Wan, Y.Y.; Nakae, S.; Iwakura, Y.; Hao, L.; et al. Memory/Effector (CD45RBlo) CD4 T Cells Are Controlled Directly by IL-10 and Cause IL-22–Dependent Intestinal Pathology. J. Exp. Med. 2011, 208, 1027–1040. [Google Scholar] [CrossRef]
- Kirchberger, S.; Royston, D.J.; Boulard, O.; Thornton, E.; Franchini, F.; Szabady, R.L.; Harrison, O.; Powrie, F. Innate Lymphoid Cells Sustain Colon Cancer through Production of Interleukin-22 in a Mouse Model. J. Exp. Med. 2013, 210, 917–931. [Google Scholar] [CrossRef]
- Aden, K.; Tran, F.; Ito, G.; Sheibani-Tezerji, R.; Lipinski, S.; Kuiper, J.W.; Tschurtschenthaler, M.; Saveljeva, S.; Bhattacharyya, J.; Häsler, R.; et al. ATG16L1 Orchestrates Interleukin-22 Signaling in the Intestinal Epithelium via cGAS–STING. J. Exp. Med. 2018, 215, 2868–2886. [Google Scholar] [CrossRef]
- Van De Bunt, M.; D’Alessio, S.; Norrild, J.C.; Kjølbye, A.L.; Jorgensen, R. P111 Efficacy of a Novel Long-Acting Lipidated Interleukin-22, Alone and in Combination with Anti-TNF Treatment, in a Murine Chronic DSS Colitis Model. J. Crohn’s Colitis 2023, 17, i276. [Google Scholar] [CrossRef]
- Wagner, F.; Mansfield, J.C.; Lekkerkerker, A.N.; Wang, Y.; Keir, M.; Dash, A.; Butcher, B.; Harder, B.; Orozco, L.D.; Mar, J.S.; et al. Dose Escalation Randomised Study of Efmarodocokin Alfa in Healthy Volunteers and Patients with Ulcerative Colitis. Gut 2023, 72, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, P.; Tsakmaki, A.; Pantazi, E.; Li, K.; Cozzetto, D.; Digby- Bell, J.; Yang, F.; Lo, J.W.; Alberts, E.; Sa, A.C.C.; et al. Interleukin-22 Regulates Neutrophil Recruitment in Ulcerative Colitis and Is Associated with Resistance to Ustekinumab Therapy. Nat. Commun. 2022, 13, 5820. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Moksnes, C.; Fernandez-Llaneza, D.; Neisen, J.; Angermann, B.R.; Gehrmann, U.; Olsson, M.; Cairns, J.; Powell, N. P879 Clinical Significance of Plasma Interleukin-22 as a Biomarker of Inflammation in Patients with Crohn’s Disease and Ulcerative Colitis. J. Crohn’s Colitis 2023, 17, i996–i997. [Google Scholar] [CrossRef]
- Garbers, C.; Hermanns, H.M.; Schaper, F.; Müller-Newen, G.; Grötzinger, J.; Rose-John, S.; Scheller, J. Plasticity and Cross-Talk of Interleukin 6-Type Cytokines. Cytokine Growth Factor Rev. 2012, 23, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.D. The Enigmatic Cytokine Oncostatin M and Roles in Disease. ISRN Inflamm. 2013, 2013, 512103. [Google Scholar] [CrossRef]
- Hermanns, H.M. Oncostatin M and Interleukin-31: Cytokines, Receptors, Signal Transduction and Physiology. Cytokine Growth Factor Rev. 2015, 26, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Mirkov, M.U.; Verstockt, B.; Cleynen, I. Genetics of Inflammatory Bowel Disease: Beyond NOD2. Lancet Gastroenterol. Hepatol. 2017, 2, 224–234. [Google Scholar] [CrossRef]
- Guo, A.; Ross, C.; Chande, N.; Gregor, J.; Ponich, T.; Khanna, R.; Sey, M.; Beaton, M.; Yan, B.; Kim, R.B.; et al. High Oncostatin M Predicts Lack of Clinical Remission for Patients with Inflammatory Bowel Disease on Tumor Necrosis Factor α Antagonists. Sci. Rep. 2022, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Fornai, M.; Fornili, M.; Antonioli, L.; Benvenuti, L.; Tapete, G.; Baiano Svizzero, G.; Ceccarelli, L.; Mumolo, M.G.; Baglietto, L.; et al. Serum Oncostatin M at Baseline Predicts Mucosal Healing in Crohn’s Disease Patients Treated with Infliximab. Aliment. Pharmacol. Ther. 2020, 52, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Minar, P.; Lehn, C.; Tsai, Y.-T.; Jackson, K.; Rosen, M.J.; Denson, L.A. Elevated Pretreatment Plasma Oncostatin M Is Associated with Poor Biochemical Response to Infliximab. Crohn’s Colitis 2019, 360, otz026. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, S.; Verstockt, B.; Machiels, K.; Vancamelbeke, M.; Ferrante, M.; Cleynen, I.; De Hertogh, G.; Vermeire, S. Oncostatin M Is a Biomarker of Diagnosis, Worse Disease Prognosis, and Therapeutic Nonresponse in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chanchlani, N.; Invergo, B.M.; Anderson, M.W.; Guay, H.M.; Reppell, M.; Butler, J.W.; Goodhand, J.R.; Ahmad, T.; Kennedy, N.A.; et al. DOP28 Understanding the Molecular Mechanisms of Anti-TNF Treatment Failure in Patients with Crohn’s Disease: A Pilot Serum Proteomic Analysis of the PANTS Cohort. J. Crohn’s Colitis 2020, 14, S067–S068. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Kowalski, K.; Sandborn, W.J.; Feagan, B. Population Pharmacokinetics and Exposure–Response Analyses of Ustekinumab in Patients with Moderately to Severely Active Crohn’s Disease. Clin. Ther. 2022, 44, 1336–1355. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Jacobstein, D.; Szapary, P.; Johanns, J.; Gao, L.-L.; Davis, H.M.; Hanauer, S.B.; Feagan, B.G.; et al. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients with Crohn’s Disease. Gastroenterology 2018, 154, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Dreesen, E.; Noman, M.; Outtier, A.; Van den Berghe, N.; Aerden, I.; Compernolle, G.; Van Assche, G.; Gils, A.; Vermeire, S.; et al. Ustekinumab Exposure-Outcome Analysis in Crohn’s Disease Only in Part Explains Limited Endoscopic Remission Rates. J. Crohn’s Colitis 2019, 13, 864–872. [Google Scholar] [CrossRef]
- Battat, R.; Kopylov, U.; Bessissow, T.; Bitton, A.; Cohen, A.; Jain, A.; Martel, M.; Seidman, E.; Afif, W. Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 1427–1434.e2. [Google Scholar] [CrossRef]
- Soufflet, N.; Boschetti, G.; Roblin, X.; Cuercq, C.; Williet, N.; Charlois, A.-L.; Duclaux-Loras, R.; Danion, P.; Mialon, A.; Faure, M.; et al. Concentrations of Ustekinumab During Induction Therapy Associate with Remission in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 2610–2612. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kang, B.; Kim, E.S.; Choe, Y.H.; Kim, M.J. Comparison of Ustekinumab Trough Concentrations Measured by 2 ELISA Kits and Evaluation of Clinical Response in Crohn’s Disease. Ther. Drug. Monit. 2022, 44, 535–542. [Google Scholar] [CrossRef]
- Morita, Y.; Imai, T.; Bamba, S.; Takahashi, K.; Inatomi, O.; Miyazaki, T.; Watanabe, K.; Nakamura, S.; Yoshida, A.; Endo, Y.; et al. Clinical Relevance of Innovative Immunoassays for Serum Ustekinumab and Anti-Ustekinumab Antibody Levels in Crohn’s Disease. J. Gastroenterol. Hepatol. 2020, 35, 1163–1170. [Google Scholar] [CrossRef]
- Gómez Espín, R.; Nicolás De Prado, I.; Gil Candel, M.; González Carrión, M.; Rentero Redondo, L.; Iniesta Navalón, C. Association between Ustekinumab Trough Concentrations and Biochemical Outcomes in Patients with Crohn’s Disease. A Real Life Study. Rev. Esp. Enfermadades Dig. 2021, 113, 110–115. [Google Scholar] [CrossRef]
- Kolar, M.; Pudilova, K.; Bortlik, M.; Duricova, D.; Malickova, K.; Hruba, V.; Machkova, N.; Vanickova, R.; Mitrova, K.; Lukas, M.; et al. Mo1919—Ustekinumab Efficiency As a Higher-Line Therapy in Association with Serum Levels in Patients with Crohn’s Disease. Gastroenterology 2019, 156, S-886–S-887. [Google Scholar] [CrossRef]
- Hirayama, H.; Morita, Y.; Imai, T.; Takahashi, K.; Yoshida, A.; Bamba, S.; Inatomi, O.; Andoh, A. Ustekinumab Trough Levels Predicting Laboratory and Endoscopic Remission in Patients with Crohn’s Disease. BMC Gastroenterol. 2022, 22, 195. [Google Scholar] [CrossRef]
- McDonald, C.; Kerr, H.; Gibbons, E.; Lukose, T.; Cheriyan, D.; Harewood, G.; Patchett, S.; O’Toole, A.; Kelly, O.; Boland, K. Higher Ustekinumab Levels in Maintenance Therapy Are Associated with Greater Mucosal Healing and Mucosal Response in Crohn’s Disease: An Experience of 2 IBD Centers. Inflamm. Bowel Dis. 2023, 30, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Kawamoto, A.; Hibiya, S.; Suzuki, K.; Fujii, T.; Motobayashi, M.; Shimizu, H.; Nagahori, M.; Saito, E.; Okamoto, R.; et al. Higher Concentrations of Cytokine Blockers Are Needed to Obtain Small Bowel Mucosal Healing during Maintenance Therapy in Crohn’s Disease. Aliment. Pharmacol. Ther. 2021, 54, 1052–1060. [Google Scholar] [CrossRef]
- Van den Berghe, N.; Verstockt, B.; Vermeire, S.; Thomas, D.; Declerck, P. Higher Drug Exposure during the First 24 Weeks of Ustekinumab Treatment Is Associated with Endoscopic Remission in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 838–840.e2. [Google Scholar] [CrossRef]
- Painchart, C.; Brabant, S.; Duveau, N.; Nachury, M.; Desreumaux, P.; Branche, J.; Gérard, R.; Prevost, C.L.D.; Wils, P.; Lambin, T.; et al. Ustekinumab Serum Trough Levels May Identify Suboptimal Responders to Ustekinumab in Crohn’s Disease. Dig. Dis. Sci. 2020, 65, 1445–1452. [Google Scholar] [CrossRef]
- Proietti, E.; Pauwels, R.W.M.; van der Woude, C.J.; Doukas, M.; Oudijk, L.; Peppelenbosch, M.P.; Grohmann, U.; Crombag, M.-R.B.S.; de Vries, A.C.; Fuhler, G.M. Ustekinumab Tissue and Serum Levels in Patients with Crohn’s Disease Are Closely Correlated though Not Consistently Associated with Objective Response after Induction. Inflamm. Bowel Dis. 2023, 29, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Straatmijer, T.; Biemans, V.B.C.; Moes, D.J.A.R.; Hoentjen, F.; Ter Heine, R.; Maljaars, P.W.J.; Theeuwen, R.; Pierik, M.; Duijvestein, M.; van der Meulen-de Jong, A.E.; et al. Ustekinumab Trough Concentrations Are Associated with Biochemical Outcomes in Patients with Crohn’s Disease. Dig. Dis. Sci. 2023, 68, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Mechie, N.-C.; Burmester, M.; Mavropoulou, E.; Pilavakis, Y.; Kunsch, S.; Ellenrieder, V.; Amanzada, A. Evaluation of Ustekinumab Trough Levels during Induction and Maintenance Therapy with Regard to Disease Activity Status in Difficult to Treat Crohn Disease Patients. Medicine 2021, 100, e25111. [Google Scholar] [CrossRef] [PubMed]
- Liefferinckx, C.; Verstockt, B.; Gils, A.; Noman, M.; Van Kemseke, C.; Macken, E.; De Vos, M.; Van Moerkercke, W.; Rahier, J.-F.; Bossuyt, P.; et al. Long-Term Clinical Effectiveness of Ustekinumab in Patients with Crohn’s Disease Who Failed Biologic Therapies: A National Cohort Study. J. Crohn’s Colitis 2019, 13, 1401–1409. [Google Scholar] [CrossRef]





| n = 84 | ||
|---|---|---|
| Age at diagnosis (years) | Median (IQR) | 27 (19–40) |
| Age at inclusion (years) | Mean (SD) | 43 (14) |
| Female | n (%) | 32 (38.1%) |
| Disease duration (years) | Median (IQR) | 12 (7–21) |
| Follow-up (months) | Median (IQR) | 15 (13–16) |
| Active smoking | n (%) | 10 (11.9%) |
| Disease activity | ||
| SES-CD score | Median (IQR) | 7 (3.00–13.00) |
| HBI score | Median (IQR) | 5 (2–9) |
| CRP above the upper normal limit | n (%) | 38 (45.2%) |
| Faecal calprotectin (mg/kg) | Median (IQR) | 1000 (452–1900) |
| Age at onset | ||
| Below 16 years | n (%) | 15 (17.8%) |
| Between 16 and 40 years | n (%) | 47 (55.9%) |
| Above 40 years | n (%) | 22 (26.2%) |
| Disease location | ||
| Ileum | n (%) | 17 (20.2%) |
| Colon | n (%) | 18 (21.4%) |
| Ileocolonic | n (%) | 40 (47.6%) |
| Additional upper GI | n (%) | 9 (10.7%) |
| Disease behaviour | ||
| Inflammatory | n (%) | 22 (26.2%) |
| Stricturing | n (%) | 31 (36.9%) |
| Penetrating | n (%) | 33 (39.3%) |
| Perianal disease | n (%) | 23 (27.4%) |
| Extraintestinal manifestations | n (%) | 42 (50%) |
| Prior intestinal resection | n (%) | 46 (54.8%) |
| Prior perianal fistula surgical intervention | n (%) | 23 (27.4%) |
| Prior treatment | ||
| 1 anti-TNF | n (%) | 81 (96.4%) |
| ≥2 anti-TNF | n (%) | 58 (69.0%) |
| Vedolizumab | n (%) | 42 (50.0%) |
| Both vedolizumab and anti-TNF | n (%) | 39 (46.4%) |
| Concomitant treatment | ||
| Oral prednisone | n (%) | 19 (22.6%) |
| Thiopurine | n (%) | 8 (9.5%) |
| Methotrexate | n (%) | 2 (2.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertin, L.; Barberio, B.; Gubbiotti, A.; Bertani, L.; Costa, F.; Ceccarelli, L.; Visaggi, P.; Bodini, G.; Pasta, A.; Sablich, R.; et al. Association between Ustekinumab Trough Levels, Serum IL-22, and Oncostatin M Levels and Clinical and Biochemical Outcomes in Patients with Crohn’s Disease. J. Clin. Med. 2024, 13, 1539. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13061539
Bertin L, Barberio B, Gubbiotti A, Bertani L, Costa F, Ceccarelli L, Visaggi P, Bodini G, Pasta A, Sablich R, et al. Association between Ustekinumab Trough Levels, Serum IL-22, and Oncostatin M Levels and Clinical and Biochemical Outcomes in Patients with Crohn’s Disease. Journal of Clinical Medicine. 2024; 13(6):1539. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13061539
Chicago/Turabian StyleBertin, Luisa, Brigida Barberio, Alessandro Gubbiotti, Lorenzo Bertani, Francesco Costa, Linda Ceccarelli, Pierfrancesco Visaggi, Giorgia Bodini, Andrea Pasta, Renato Sablich, and et al. 2024. "Association between Ustekinumab Trough Levels, Serum IL-22, and Oncostatin M Levels and Clinical and Biochemical Outcomes in Patients with Crohn’s Disease" Journal of Clinical Medicine 13, no. 6: 1539. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13061539