Facile Synthesis of Polyacrylic Acid/Graphene Oxide Composite Hydrogel Electrolyte for High-Performance Flexible Supercapacitors
Abstract
:1. Introduction
2. Experimental
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Electrochemical Measurements
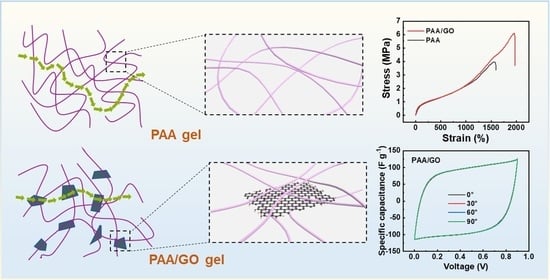
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


| Stress (MPa) | Strain (%) | Ionic Conductivity (10−2 S cm−1) | Reference | |
|---|---|---|---|---|
| S-PAA | 2.2 | 1100% | 1.5 | [18] |
| VSNPs-PAA | 0.12 | 3700% | 0.75 | [21] |
| Fe3+/PAA | 0.4 | 700% | 9 | [22] |
| Li-AG/PAM DN | 1.1 | 2780% | 4.1 | [42] |
| BC-reinforced PAM | 0.33 | 1300% | 12.5 | [43] |
| PK10 AGPE | 2.22 | 1243% | 21 | [44] |
| B-PVA/KCl/GO | \ | \ | 4.75 | [45] |
| PAA/GO | 6.1 | 1950% | 16.81 | This work |
References
- Zhang, J.; Luo, J.; Guo, Z.; Liu, Z.; Duan, C.; Dou, S.; Yuan, Q.; Liu, P.; Ji, K.; Zeng, C.; et al. Ultrafast Manufacturing of Ultrafine Structure to Achieve An Energy Density of Over 120 Wh kg −1 in Supercapacitors. Adv. Energy Mater. 2022, 27, 2203061. [Google Scholar] [CrossRef]
- Muralee Gopi, C.V.V.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kim, H.-J. Recent progress of advanced energy storage materials for flexible and wearable supercapacitor: From design and development to applications. J. Energy Storage 2020, 27, 101035. [Google Scholar] [CrossRef]
- Xiao, J.; Han, J.; Zhang, C.; Ling, G.; Kang, F.; Yang, Q.H. Dimensionality, Function and Performance of Carbon Materials in Energy Storage Devices. Adv. Energy Mater. 2021, 12, 2100775. [Google Scholar] [CrossRef]
- Yu, S.; Sun, N.; Hu, L.; Wang, L.; Zhu, Q.; Guan, Y.; Xu, B. Self-template and self-activation synthesis of nitrogen-doped hierarchical porous carbon for supercapacitors. J. Power Source 2018, 405, 132–141. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Han, Y.; Li, T. Flexible supercapacitor: Overview and outlooks. J. Energy Storage 2021, 42, 103053. [Google Scholar] [CrossRef]
- Thomas, S.A.; Patra, A.; Al-Shehri, B.M.; Selvaraj, M.; Aravind, A.; Rout, C.S. MXene based hybrid materials for supercapacitors: Recent developments and future perspectives. J. Energy Storage 2022, 55, 105765. [Google Scholar] [CrossRef]
- Swain, N.; Tripathy, A.; Thirumurugan, A.; Saravanakumar, B.; Schmidt-Mende, L.; Ramadoss, A. A brief review on stretchable, compressible, and deformable supercapacitor for smart devices. Chem. Eng. J. 2022, 446, 136876. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Ma, G.; Dong, M.; Sun, K.; Feng, E.; Peng, H.; Lei, Z. A redox mediator doped gel polymer as an electrolyte and separator for a high performance solid state supercapacitor. J. Mater. Chem. A 2015, 3, 4035–4041. [Google Scholar] [CrossRef]
- Menkin, S.; Lifshitz, M.; Haimovich, A.; Goor, M.; Blanga, R.; Greenbaum, S.G.; Goldbourt, A.; Golodnitsky, D. Evaluation of ion-transport in composite polymer-in-ceramic electrolytes. Case study of active and inert ceramics. Electrochim. Acta 2019, 304, 447–455. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, Y.; Zhang, X.; Pi, M.; Wang, X.; Zhu, R.; Cui, W.; Ran, R. Highly Deformable, Conductive Double-Network Hydrogel Electrolytes for Durable and Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 15641–15652. [Google Scholar] [CrossRef]
- Luangaramvej, P.; Dubas, S.T. Two-step polyaniline loading in polyelectrolyte complex membranes for improved pseudo-capacitor electrodes. e-Polymers 2021, 21, 194–199. [Google Scholar] [CrossRef]
- Na, R.; Huo, P.; Zhang, X.; Zhang, S.; Du, Y.; Zhu, K.; Lu, Y.; Zhang, M.; Luan, J.; Wang, G. A flexible solid-state supercapacitor based on a poly(aryl ether ketone)–poly(ethylene glycol) copolymer solid polymer electrolyte for high temperature applications. RSC Adv. 2016, 6, 65186–65195. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Jia, H.; Ng, P.F.; Chow, L.; Fei, B. Recent advances of hydrogel electrolytes in flexible energy storage devices. J. Mater. Chem. A 2021, 9, 2043–2069. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Liu, J.; Zhang, J.; Ma, X.; Huang, Y. An intrinsically compressible and stretchable all-in-one configured supercapacitor. ChemComm 2018, 54, 6200–6203. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, Z.; Wang, C.; Yuan, Z.; Wei, L.; Pan, Y.; Mahmood, A.; Shao, Q.; Chen, Y. Flexible Zinc-Ion Hybrid Fiber Capacitors with Ultrahigh Energy Density and Long Cycling Life for Wearable Electronics. Small 2019, 15, 1903817. [Google Scholar] [CrossRef]
- Liao, H.; Zhou, F.; Zhang, Z.; Yang, J. A self-healable and mechanical toughness flexible supercapacitor based on polyacrylic acid hydrogel electrolyte. Chem. Eng. J. 2019, 357, 428–434. [Google Scholar] [CrossRef]
- Abubshait, H.A.; Saad, M.; Iqbal, S.; Abubshait, S.A.; Bahadur, A.; Raheel, M.; Alshammari, F.H.; Alwadai, N.; Alrbyawi, H.; Abourehab, M.A.S.; et al. Co-doped zinc oxide nanoparticles embedded in Polyvinylalcohol Hydrogel as solar light derived photocatalyst disinfection and removal of coloured pollutants. J. Mol. Struct. 2023, 1271, 134100. [Google Scholar] [CrossRef]
- Iqbal, S.; Javed, M.; Qamar, M.A.; Bahadur, A.; Fayyaz, M.; Akbar, A.; Alsaab, H.O.; Awwad, N.S.; Ibrahium, H.A. Synthesis of Cu-ZnO/Polyacrylic Acid Hydrogel as Visible-Light-Driven Photocatalyst for Organic Pollutant Degradation. ChemistrySelect 2022, 7, e202103694. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Huang, Y.; Zhu, M.; Pei, Z.; Wang, Z.; Xue, Q.; Xie, X.; Zhi, C. A self-healable and highly stretchable supercapacitor based on a dual crosslinked polyelectrolyte. Nat. Commun. 2015, 6, 10310. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, X.; Tang, Q.; Bao, H.; Wang, G.; Saha, P. A self-healable and easily recyclable supramolecular hydrogel electrolyte for flexible supercapacitors. J. Mater. Chem. A 2016, 4, 8769–8776. [Google Scholar] [CrossRef]
- Cao, Y.-C.; Xu, C.; Wu, X.; Wang, X.; Xing, L.; Scott, K. A poly (ethylene oxide)/graphene oxide electrolyte membrane for low temperature polymer fuel cells. J. Power Source 2011, 196, 8377–8382. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Wu, P.-F.; Zhang, M.-Q.; Ruan, W.-H.; Giannelis, E.P. Boron cross-linked graphene oxide/polyvinyl alcohol nanocomposite gel electrolyte for flexible solid-state electric double layer capacitor with high performance. Electrochim. Acta 2014, 132, 103–111. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Zhu, Q.; Sun, N.; Anasori, B.; Hu, L.; Wang, F.; Guan, Y.; Gogotsi, Y. Reduced graphene oxide as a multi-functional conductive binder for supercapacitor electrodes. Energy Storage Mater. 2018, 12, 128–136. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Y.; Liu, Y.-T.; Zhu, Q.; Shen, J.; Liu, H.; Zhou, S.; Xu, B. Facile synthesis of free-standing, flexible hard carbon anode for high-performance sodium ion batteries using graphene as a multi-functional binder. Carbon 2018, 137, 475–483. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Huang, Y.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional Energy Storage and Conversion Devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Gai, G. Recent Progress of Graphene-Containing Polymer Hydrogels: Preparations, Properties, and Applications. Macromol. Mater. Eng. 2017, 302, 1700184. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, Z.; Cao, L.; Zhang, X.L.; Sun, C.; Wang, D.-W. Graphene oxide: An emerging electromaterial for energy storage and conversion. J. Energy Chem. 2021, 55, 323–344. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Han, Q.; Chen, L.; Li, W.; Zhou, Z.; Fang, Z.; Xu, Z.; Qian, X. Self-assembled three-dimensional double network graphene oxide/polyacrylic acid hybrid aerogel for removal of Cu(2+) from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 34438–34447. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Piloto, C.; Shafiei, M.; Khan, H.; Gupta, B.; Tesfamichael, T.; Motta, N. Sensing performance of reduced graphene oxide-Fe doped WO3 hybrids to NO2 and humidity at room temperature. Appl. Surf. Sci. 2018, 434, 126–133. [Google Scholar] [CrossRef]
- Kong, W.; Yue, Q.; Li, Q.; Gao, B. Adsorption of Cd(2+) on GO/PAA hydrogel and preliminary recycle to GO/PAA-CdS as efficient photocatalyst. Sci. Total Environ. 2019, 668, 1165–1174. [Google Scholar] [CrossRef]
- Feng, Z.; Feng, C.; Chen, N.; Lu, W.; Wang, S. Preparation of composite hydrogel with high mechanical strength and reusability for removal of Cu(II) and Pb(II) from water. Sep. Purif. Technol. 2022, 300, 121894. [Google Scholar] [CrossRef]
- Wang, S.H.; Hou, S.S.; Kuo, P.L.; Teng, H. Poly(ethylene oxide)-co-poly(propylene oxide)-based gel electrolyte with high ionic conductivity and mechanical integrity for lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 8477–8485. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Zhang, L.; Zhang, T.; Huang, Y.; Chen, Y. A High-Performance Graphene Oxide-Doped Ion Gel as Gel Polymer Electrolyte for All-Solid-State Supercapacitor Applications. Adv. Funct. Mater. 2013, 23, 3353–3360. [Google Scholar] [CrossRef]
- Fan, X.; Lu, Y.; Xu, H.; Kong, X.; Wang, J. Reversible redox reaction on the oxygen-containing functional groups of an electrochemically modified graphite electrode for the pseudo-capacitance. J. Mater. Chem. 2011, 21, 18753. [Google Scholar] [CrossRef]
- Islam, T.; Hasan, M.M.; Shah, S.S.; Karim, M.R.; Al-Mubaddel, F.S.; Zahir, M.H.; Dar, M.A.; Hossain, M.D.; Aziz, M.A.; Ahammad, A.J.S. High yield activated porous coal carbon nanosheets from Boropukuria coal mine as supercapacitor material: Investigation of the charge storing mechanism at the interfacial region. J Energy Storage 2020, 32, 101908. [Google Scholar] [CrossRef]
- Lin, T.; Shi, M.; Huang, F.; Peng, J.; Bai, Q.; Li, J.; Zhai, M. One-Pot Synthesis of a Double-Network Hydrogel Electrolyte with Extraordinarily Excellent Mechanical Properties for a Highly Compressible and Bendable Flexible Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 29684–29693. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, L.; Liu, R.; He, H.; Hao, J.; Lu, Y.; Wang, Y.; Liang, G.; Yuan, G.; Guo, Z. Engineering Textile Electrode and Bacterial Cellulose Nanofiber Reinforced Hydrogel Electrolyte to Enable High-Performance Flexible All-Solid-State Supercapacitors. Adv. Energy Mater. 2021, 11, 2003010. [Google Scholar] [CrossRef]
- Hu, X.; Fan, L.; Qin, G.; Shen, Z.; Chen, J.; Wang, M.; Yang, J.; Chen, Q. Flexible and low temperature resistant double network alkaline gel polymer electrolyte with dual-role KOH for supercapacitor. J. Power Source 2019, 414, 201–209. [Google Scholar] [CrossRef]
- Peng, H.; Lv, Y.; Wei, G.; Zhou, J.; Gao, X.; Sun, K.; Ma, G.; Lei, Z. A flexible and self-healing hydrogel electrolyte for smart supercapacitor. J. Power Source 2019, 431, 210–219. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Yu, Z.; Soomro, R.A.; Sun, N. Facile Synthesis of Polyacrylic Acid/Graphene Oxide Composite Hydrogel Electrolyte for High-Performance Flexible Supercapacitors. Coatings 2023, 13, 382. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13020382
Xin Y, Yu Z, Soomro RA, Sun N. Facile Synthesis of Polyacrylic Acid/Graphene Oxide Composite Hydrogel Electrolyte for High-Performance Flexible Supercapacitors. Coatings. 2023; 13(2):382. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13020382
Chicago/Turabian StyleXin, Yue, Zhaoxin Yu, Razium Ali Soomro, and Ning Sun. 2023. "Facile Synthesis of Polyacrylic Acid/Graphene Oxide Composite Hydrogel Electrolyte for High-Performance Flexible Supercapacitors" Coatings 13, no. 2: 382. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings13020382