Binder-Free CNT-Modified Excellent Electrodes for All-Vanadium Redox Flow Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CNT-GFs
2.2. Characterization of Electrodes
2.3. Evaluation of Electrochemical Performance
2.4. Measurement of VRFB Performance
3. Results and Discussions
3.1. Characteristics of CNT-GFs
3.2. Electrochemical Characteristics of CNT-GFs
3.3. VRFB Performance
4. Conclusions
- A CNT homogeneous solution was successfully prepared using NMP solvent with the aid of probe sonication, followed by the dip-coating method to coat CNTs on H-GF. FESEM and Raman analysis confirmed the successful deposition of CNTs on H-GF, resulting in improved wettability, as demonstrated by super hydrophilic behavior.
- The CNT-GF exhibited lower resistance and higher cathodic and anodic peak currents for both vanadium redox pairs. These results indicate enhanced reaction kinetics of VO2+/VO2+ and V3+/V2+ whether the CNT-GF serves in the positive or negative side of a VRFB.
- At a current density of 100 mA cm−2, VRFBs with N-CNT-GF or P-CNT-GF demonstrated energy efficiencies of 81% or 82%, respectively, whereas the VRFB with B-H-GFs showed an energy efficiency of 79%. At a high current density of 200 mA cm−2, P-CNT-GF delivered an energy efficiency of 72%, a 6% improvement over B-H-GF. This improvement is attributed to lower polarization losses and higher electrode reversibility.
- Coating CNTs on H-GFs improved the electrolyte utilization of the VRFB, increasing it from 43% to around 60%, a significant improvement of 17%.
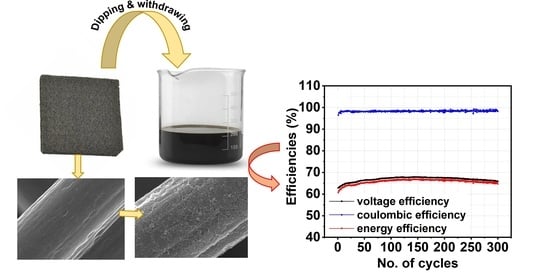
- The VRFB exhibited stable operation for 300 cycles at 200 mA cm−2, demonstrating the durability of the CNT-GF electrode.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Düerkop, D.; Widdecke, H.; Schilde, C.; Kunz, U.; Schmiemann, A. Polymer membranes for all-vanadium redox flow batteries: A Review. Membranes 2021, 11, 214. [Google Scholar] [CrossRef]
- Hu, G.; Jing, M.; Wang, D.-W.; Sun, Z.; Xu, C.; Ren, W.; Cheng, H.-M.; Yan, C.; Fan, X.; Li, F. A gradient bi-functional graphene-based modified electrode for vanadium redox flow batteries. Energy Storage Mater. 2018, 13, 66–71. [Google Scholar] [CrossRef]
- Jiang, F.; He, Z.; Guo, D.; Zhou, X. Carbon aerogel modified graphite felt as advanced electrodes for vanadium redox flow batteries. J. Power Sources 2019, 440, 227114. [Google Scholar] [CrossRef]
- Lu, M.-Y.; Yang, W.-W.; Zhang, Z.-K.; Yang, Y.-J.; Xu, Q. Lead-modified graphite felt electrode with improved VO2+/VO2+ electrochemical activity for vanadium redox flow battery. Electrochim. Acta 2022, 428, 140900. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Tsai, P.-H.; Hsu, N.-Y.; Chen, Y.-S. Effect of compression ratio of graphite felts on the performance of an all-vanadium redox flow battery. Energies 2019, 12, 313. [Google Scholar] [CrossRef]
- Cheng, D.; Li, Y.; Zhang, J.; Tian, M.; Wang, B.; He, Z.; Dai, L.; Wang, L. Recent advances in electrospun carbon fiber electrode for vanadium redox flow battery: Properties, structures, and perspectives. Carbon 2020, 170, 527–542. [Google Scholar] [CrossRef]
- Greco, K.V.; Forner-Cuenca, A.; Mularczyk, A.; Eller, J.; Brushett, F.R. Elucidating the nuanced effects of thermal pretreatment on carbon paper electrodes for vanadium redox flow batteries. ACS Appl. Mater. Interfaces 2018, 10, 44430–44442. [Google Scholar] [CrossRef] [PubMed]
- Mazúr, P.; Mrlík, J.; Beneš, J.; Pocedič, J.; Vrána, J.; Dundálek, J.; Kosek, J. Performance evaluation of thermally treated graphite felt electrodes for vanadium redox flow battery and their four-point single cell characterization. J. Power Sources 2018, 380, 105–114. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chou, H.-W.; Hsu, N.-Y.; Chen, Y.-S. Development of integrally molded bipolar plates for all-vanadium redox flow batteries. Energies 2016, 9, 350. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Shen, Y.; Xu, P.; Lu, L.; Fu, J.; Zhao, H. Treatment of graphite felt by modified Hummers method for the positive electrode of vanadium redox flow battery. Electrochim. Acta 2014, 138, 264–269. [Google Scholar] [CrossRef]
- Li, Q.; Dong, Q.; Zhang, T.; Xue, Z.; Li, J.; Wang, Z.; Sun, H. Performance of room-temperature activated tubular polypyrrole modified graphite felt composite electrode in vanadium redox flow battery. Electrochim. Acta 2022, 409, 139970. [Google Scholar] [CrossRef]
- Xing, F.; Liu, T.; Yin, Y.; Bi, R.; Zhang, Q.; Yin, L.; Li, X. Highly active hollow porous carbon spheres@graphite felt composite electrode for high power density vanadium flow batteries. Adv. Funct. Mater. 2022, 32, 2111267. [Google Scholar] [CrossRef]
- Ma, Q.; Deng, Q.; Sheng, H.; Ling, W.; Wang, H.-R.; Jiao, H.-W.; Wu, X.-W.; Zhou, W.-X.; Zeng, X.-X.; Yin, Y.-X.; et al. High electro-catalytic graphite felt/MnO2 composite electrodes for vanadium redox flow batteries. Sci. China Chem. 2018, 61, 732–738. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, Y.; Xi, J.; Qiu, X.; Chen, L. ZrO2-nanoparticle-modified graphite felt: Bifunctional effects on vanadium flow batteries. ACS Appl. Mater. Interfaces 2016, 8, 15369–15378. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.-C.; Li, J.; Yang, Y.-J.; Ren, Y.-J.; Dai, L.; Gao, J.-Y.; Wang, L.; Ye, J.-Y.; He, Z.-X. Ultrafine SnO2 in situ modified graphite felt derived from metal–organic framework as a superior electrode for vanadium redox flow battery. Rare Met. 2023, 42, 1214–1226. [Google Scholar] [CrossRef]
- Madian, M.; Eychmüller, A.; Giebeler, L. Current advances in TiO2-based nanostructure electrodes for high performance lithium ion batteries. Batteries 2018, 4, 7. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Lu, L.; Zhao, H.; Fu, J.; Shen, Y.; Xu, P.; Dong, Y. PbO2-modified graphite felt as the positive electrode for an all-vanadium redox flow battery. J. Power Sources 2014, 250, 274–278. [Google Scholar] [CrossRef]
- Tang, Z.; Zou, J.; Zhang, D.; Chen, X.; Yu, Y.; Huang, K.; Xu, Z. TixOy loaded carbon felt as high performance negative for vanadium redox flow battery. J. Power Sources 2023, 566, 232925. [Google Scholar] [CrossRef]
- Jiang, Q.; Ren, Y.; Yang, Y.; Wang, L.; Dai, L.; He, Z. Recent advances in carbon-based electrocatalysts for vanadium redox flow battery: Mechanisms, properties, and perspectives. Compos. Part B Eng. 2022, 242, 110094. [Google Scholar] [CrossRef]
- Deng, Q.; Huang, P.; Zhou, W.-X.; Ma, Q.; Zhou, N.; Xie, H.; Ling, W.; Zhou, C.-J.; Yin, Y.-X.; Wu, X.-W.; et al. A high-performance composite electrode for vanadium redox flow batteries. Adv. Energy Mater. 2017, 7, 1700461. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, Y.; Gao, J.; Li, J.; Zhu, W.; Dai, L.; Liu, Y.; Wang, L.; He, Z. Controlled synthesis of carbon nanonetwork wrapped graphite felt electrodes for high-performance vanadium redox flow battery. Electrochim. Acta 2022, 431, 141135. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. The electrochemical catalytic activity of single-walled carbon nanotubes towards VO2+/VO2+ and V3+/V2+ redox pairs for an all vanadium redox flow battery. Electrochim. Acta 2012, 79, 102–108. [Google Scholar] [CrossRef]
- Yang, D.-S.; Lee, J.Y.; Jo, S.-W.; Yoon, S.J.; Kim, T.-H.; Hong, Y.T. Electrocatalytic activity of nitrogen-doped CNT graphite felt hybrid for all-vanadium redox flow batteries. Int. J. Hydrog. Energy 2018, 43, 1516–1522. [Google Scholar] [CrossRef]
- Tai, Z.; Ju, D.; Sato, S.; Hanawa, K. Discrete coating of CNT on carbon fiber surfaces and the effect on improving the electrochemical performance of VRFB systems. Coatings 2021, 11, 736. [Google Scholar] [CrossRef]
- Ngoma, M.M.; Mathaba, M.; Moothi, K. Effect of carbon nanotubes loading and pressure on the performance of a polyethersulfone (PES)/carbon nanotubes (CNT) membrane. Sci. Rep. 2021, 11, 23805. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Tzedakis, T. Facile chemical activation of graphite felt by KMnO4 acidic solution for vanadium redox flow batteries. Appl. Surf. Sci. 2020, 528, 146808. [Google Scholar] [CrossRef]
- Li, Q.; Bai, A.; Zhang, T.; Li, S.; Sun, H. Dopamine-derived nitrogen-doped carboxyl multiwalled carbon nanotube-modified graphite felt with improved electrochemical activity for vanadium redox flow batteries. R. Soc. Open Sci. 2020, 7, 200402. [Google Scholar] [CrossRef]
- Duan, W.; Li, B.; Lu, D.; Wei, X.; Nie, Z.; Murugesan, V.; Kizewski, J.P.; Hollas, A.; Reed, D.; Sprenkle, V. Towards an all-vanadium redox flow battery with higher theoretical volumetric capacities by utilizing the VO2+/V3+ couple. J. Energy Chem. 2018, 27, 1381–1385. [Google Scholar] [CrossRef]
- Dong, L.; Jiang, W.; Pan, K.; Zhang, L. Rational Design of TiO2@g-C3N4/CNT Composite Separator for High Performance Lithium-Sulfur Batteries to Promote the Redox Kinetics of Polysulfide. Nanomaterials 2023, 13, 3084. [Google Scholar] [CrossRef]
- Pan, J.; Huang, M.; Li, X.; Wang, S.; Li, W.; Ma, T.; Xie, X.; Ramani, V. The performance of all vanadium redox flow batteries at below-ambient temperatures. Energy 2016, 107, 784–790. [Google Scholar] [CrossRef]
- Sundriyal, S.; Shrivastav, V.; Kaur, H.; Mishra, S.; Deep, A. High-performance symmetrical supercapacitor with a combination of a ZIF-67/rGO composite electrode and a redox additive electrolyte. ACS Omega 2018, 3, 17348–17358. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H. Electrokinetic parameters of a vanadium redox flow battery with varying temperature and electrolyte flow rate. Renew. Energy 2019, 138, 284–291. [Google Scholar] [CrossRef]
- Sankar, A.; Michos, I.; Dutta, I.; Dong, J.; Angelopoulos, A.P. Enhanced vanadium redox flow battery performance using graphene nanoplatelets to decorate carbon electrodes. J. Power Sources 2018, 387, 91–100. [Google Scholar] [CrossRef]
- Wei, G.; Jia, C.; Liu, J.; Yan, C. Carbon felt supported carbon nanotubes catalysts composite electrode for vanadium redox flow battery application. J. Power Sources 2012, 220, 185–192. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Mousavihashemi, S.; Murcia-López, S.; Flox, C.; Andreu, T.; Morante, J.R. High-power positive electrode based on synergistic effect of N- and WO3 -decorated carbon felt for vanadium redox flow batteries. Carbon 2018, 136, 444–453. [Google Scholar] [CrossRef]
- Park, M.; Jung, Y.-j.; Kim, J.; Lee, H.i.; Cho, J. Synergistic effect of carbon nanofiber/nanotube composite catalyst on carbon felt electrode for high-performance all-vanadium redox flow battery. Nano Lett. 2013, 13, 4833–4839. [Google Scholar] [CrossRef]
- Noh, C.; Moon, S.; Chung, Y.; Kwon, Y. Chelating functional group attached to carbon nanotubes prepared for performance enhancement of vanadium redox flow battery. J. Mater. Chem. A 2017, 5, 21334–21342. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Lin, X.; Yang, L.; Yu, A. Study on nitrogen-doped carbon nanotubes for vanadium redox flow battery application. Int. J. Electrochem. Sci. 2016, 11, 665–674. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Shih, Y.-C.; Chen, J.-Y.; Lin, G.-Y.; Hsu, N.-Y.; Chou, Y.-S.; Wang, C.-H. High efficiency of bamboo-like carbon nanotubes on functionalized graphite felt as electrode in vanadium redox flow battery. RSC Adv. 2016, 6, 102068–102075. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.; Yeo, G.; Ko, M.; Jang, H. Designing CNT-implanted graphite felt as a sustainable electron network for long-cycling of vanadium redox flow batteries. Carbon 2023, 206, 1–6. [Google Scholar] [CrossRef]







| Electrode Type | −Ipc (A) | Ipa (A) | −Ipc/Ipa | Er (V) | Eo (V) | E (ΔV) |
|---|---|---|---|---|---|---|
| H-GF | 0.193 | 0.322 | 0.59 | 0.54 | 1.52 | 0.98 |
| CNT-GF | 0.263 | 0.396 | 0.66 | 0.46 | 1.57 | 1.11 |
| Electrode Type | Ioc (mA cm−2) | Ioa (mA cm−2) | αc | αa |
|---|---|---|---|---|
| H-GF | 1.22 | 3.03 | 0.07 | 0.052 |
| CNT-GF | 1.27 | 3.12 | 0.11 | 0.056 |
| Current Density | Voltage Efficiency | Coulombic Efficiency | Energy Efficiency |
|---|---|---|---|
| (mA cm−2) | (%) | (%) | (%) |
| B-H-GF | |||
| 100 | 82.48 | 95.85 | 79.05 |
| 150 | 75.13 | 97.23 | 73.05 |
| 200 | 67.5 | 97.97 | 66.13 |
| 100 | 83.10 | 96.48 | 80.16 |
| N-CNT-GF | |||
| 100 | 82.78 | 98.49 | 81.53 |
| 150 | 76.52 | 99.15 | 75.87 |
| 200 | 69.08 | 99.57 | 68.78 |
| 100 | 83.65 | 98.95 | 82.76 |
| P-CNT-GF | |||
| 100 | 85.06 | 96.65 | 82.21 |
| 150 | 79.13 | 97.49 | 77.16 |
| 200 | 72.97 | 98.09 | 71.57 |
| 100 | 85.85 | 96.80 | 83.10 |
| No. | Electrode | Cell Size (cm2) | Electrolyte | Current Density (mA cm−2) | Energy Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| 1. | HAA-CNT 1 | 4 × 4 | 1.5 M VOSO4 + 2 M H2SO4 | 120 | 77.5 | [38] |
| 2. | Nitrogen doped—CNT/CF | 6 × 8 | 0.9 M V(III) + 0.8 M V(IV) + 2 M H2SO4 | 40 | 76.3 | [39] |
| 3. | B-CNT/TA-GF 2 | 5 × 5 | 5 mM V2SO4 in 2 M | 80 | 76.8 | [40] |
| 4. | CNT-GF | 5 × 5 | 2 M VOSO4 + 3 M H2SO4 | 50 | 86.9 | [41] |
| 5. | Nitrogen doped CNT/GF(Fe) | 3 × 3 | 0.1 M VOSO4 + 3.0 M H2SO4 | 150 | 69 | [24] |
| 6. | MWCNTs | 6 × 6 | 1.5 M VOSO4 + 2 M H2SO4 | 50 | 82 | [35] |
| 7. | P-CNT-GF | 5 × 5 | 1.684 M VOSO4 + 4.397 M H2SO4 | 100 | 82 | This work |
| 8. | P-CNT-GF | 5 × 5 | 1.684 M VOSO4 + 4.397 M H2SO4 | 150 | 77 | This work |
| 9. | P-CNT-GF | 5 × 5 | 1.684 M VOSO4 + 4.397 M H2SO4 | 200 | 72 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, N.; Singh, P.; Chen, Y.-S. Binder-Free CNT-Modified Excellent Electrodes for All-Vanadium Redox Flow Batteries. Nanomaterials 2024, 14, 767. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14090767
Devi N, Singh P, Chen Y-S. Binder-Free CNT-Modified Excellent Electrodes for All-Vanadium Redox Flow Batteries. Nanomaterials. 2024; 14(9):767. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14090767
Chicago/Turabian StyleDevi, Nitika, Prabhakar Singh, and Yong-Song Chen. 2024. "Binder-Free CNT-Modified Excellent Electrodes for All-Vanadium Redox Flow Batteries" Nanomaterials 14, no. 9: 767. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14090767