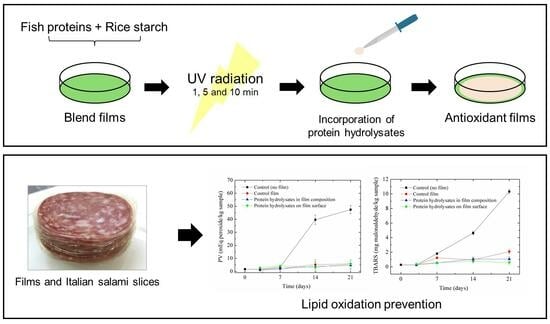
UV Radiation and Protein Hydrolysates in Bio-Based Films: Impacts on Properties and Italian Salami Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Hydrolysate Preparation
2.3. Film Production and UV Radiation Treatment
2.4. Film Evaluation
2.4.1. Packaging Characteristics
2.4.2. Microstructure and Thermal Properties
2.4.3. Antioxidant Properties of Films
2.5. Film Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Packaging Characteristics of UV-Radiated Films
3.2. Film Microstructure and Thermal Properties
3.3. Antioxidant Properties of Films Incorporated with Protein Hydrolysates
3.4. Films’ Effectiveness in Preventing Lipid Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, N. Proteins Isolates and Hydrolysates: Structure-function Relation, Production, Bioactivities and Applications for Traditional and Modern High Nutritional Value-added Food Products. Int. J. Food Sci. Technol. 2022, 57, 5567–5570. [Google Scholar] [CrossRef]
- López-Moreno, M.; Jiménez-Moreno, E.; Márquez Gallego, A.; Vera Pasamontes, G.; Uranga Ocio, J.A.; Garcés-Rimón, M.; Miguel-Castro, M. Red Quinoa Hydrolysates with Antioxidant Properties Improve Cardiovascular Health in Spontaneously Hypertensive Rats. Antioxidants 2023, 12, 1291. [Google Scholar] [CrossRef] [PubMed]
- Riolo, K.; Rotondo, A.; La Torre, G.L.; Marino, Y.; Franco, G.A.; Crupi, R.; Fusco, R.; Di Paola, R.; Oliva, S.; De Marco, G.; et al. Cytoprotective and Antioxidant Effects of Hydrolysates from Black Soldier Fly (Hermetia illucens). Antioxidants 2023, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, P.J.; Batista, I.; Pires, C.; Bandarra, N.M.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Antioxidant Activity of Protein Hydrolysates Obtained from Discarded Mediterranean Fish Species. Food Res. Int. 2014, 65, 469–476. [Google Scholar] [CrossRef]
- Da Rocha, M.; Alemán, A.; Baccan, G.C.; López-Caballero, M.E.; Gómez-Guillén, C.; Montero, P.; Prentice, C. Anti-Inflammatory, Antioxidant, and Antimicrobial Effects of Underutilized Fish Protein Hydrolysate. J. Aquat. Food Prod. Technol. 2018, 27, 592–608. [Google Scholar] [CrossRef]
- López-Cano, A.A.; Martínez-Aguilar, V.; Peña-Juárez, M.G.; López-Esparza, R.; Delgado-Alvarado, E.; Gutiérrez-Castañeda, E.J.; Del Angel-Monroy, M.; Pérez, E.; Herrera-May, A.L.; Gonzalez-Calderon, J.A. Chemically Modified Nanoparticles for Enhanced Antioxidant and Antimicrobial Properties with Cinnamon Essential Oil. Antioxidants 2023, 12, 2057. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Bangar, S.P.; Šimat, V.; Ozogul, F. Chitosan and Gelatine Biopolymer-based Active/Biodegradable Packaging for the Preservation of Fish and Fishery Products. Int. J. Food Sci. Technol. 2023, 58, 854–861. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Characterization of Active Films of Chitosan Containing Nettle Urtica Dioica L. Extract: Spectral and Water Properties, Microstructure, and Antioxidant Activity. Int. J. Biol. Macromol. 2023, 253, 127318. [Google Scholar] [CrossRef] [PubMed]
- Wyrwa, J.; Barska, A. Innovations in the Food Packaging Market: Active Packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Vilarinho, F.; Andrade, M.; Buonocore, G.G.; Stanzione, M.; Vaz, M.F.; Sanches Silva, A. Monitoring Lipid Oxidation in a Processed Meat Product Packaged with Nanocomposite Poly(Lactic Acid) Film. Eur. Polym. J. 2018, 98, 362–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Simpson, B.K.; Dumont, M.-J. Effect of Beeswax and Carnauba Wax Addition on Properties of Gelatin Films: A Comparative Study. Food Biosci. 2018, 26, 88–95. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Zhang, T.; Teng, B. A High-Performance Food Package Material Prepared by the Synergistic Crosslinking of Gelatin with Polyphenol–Titanium Complexes. Antioxidants 2024, 13, 167. [Google Scholar] [CrossRef]
- Lopes, A.C.; Klosowski, A.B.; Carvalho, B.M.; Olivato, J.B. Application and Characterisation of Industrial Brewing By-products in Biodegradable Starch-based Expanded Composites. Int. J. Food Sci. Technol. 2022, 57, 5523–5531. [Google Scholar] [CrossRef]
- Chaari, M.; Elhadef, K.; Akermi, S.; Ben Akacha, B.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Sarkar, T.; Shariati, M.A.; Rebezov, M.; et al. Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract. Antioxidants 2022, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- Peerzada Gh, J.; Sinclair, B.J.; Perinbarajan, G.K.; Dutta, R.; Shekhawat, R.; Saikia, N.; Chidambaram, R.; Mossa, A.-T. An Overview on Smart and Active Edible Coatings: Safety and Regulations. Eur. Food Res. Technol. 2023, 249, 1935–1952. [Google Scholar] [CrossRef]
- Romani, V.P.; Prentice-Hernández, C.; Martins, V.G. Active and Sustainable Materials from Rice Starch, Fish Protein and Oregano Essential Oil for Food Packaging. Ind. Crops Prod. 2017, 97, 268–274. [Google Scholar] [CrossRef]
- Romani, V.P.; Hernández, C.P.; Martins, V.G. Pink Pepper Phenolic Compounds Incorporation in Starch/Protein Blends and Its Potential to Inhibit Apple Browning. Food Packag. Shelf Life 2018, 15, 151–158. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Fitzgerald, M.A.; Kontogiorgos, V. Tuning the Mechanical Properties of Pectin Films with Polyphenol-Rich Plant Extracts. Int. J. Biol. Macromol. 2023, 253, 127536. [Google Scholar] [CrossRef] [PubMed]
- Romani, V.P.; Olsen, B.; Pinto Collares, M.; Meireles Oliveira, J.R.; Prentice, C.; Guimarães Martins, V. Plasma Technology as a Tool to Decrease the Sensitivity to Water of Fish Protein Films for Food Packaging. Food Hydrocoll. 2019, 94, 210–216. [Google Scholar] [CrossRef]
- Drobota, M.; Gradinaru, L.M.; Ciobanu, C.; Stoica, I. Collagen Immobilization on Poly(Ethylene Terephthalate) and Polyurethane Films after UV Functionalization. J. Adhes. Sci. Technol. 2015, 29, 2208–2219. [Google Scholar] [CrossRef]
- Kowalonek, J. Studies of Chitosan/Pectin Complexes Exposed to UV Radiation. Int. J. Biol. Macromol. 2017, 103, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Askari, G.; EmamDjomeh, Z.; Salami, M. UV-Irradiated Gelatin-Chitosan Bio-Based Composite Film, Physiochemical Features and Release Properties for Packaging Applications. Int. J. Biol. Macromol. 2020, 147, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Almasi, H.; Pirouzifard, M.K. Effect of Ultraviolet Radiation on Morphological and Physicochemical Properties of Sesame Protein Isolate Based Edible Films. Food Hydrocoll. 2018, 85, 136–143. [Google Scholar] [CrossRef]
- Schmid, M.; Held, J.; Hammann, F.; Schlemmer, D.; Noller, K. Effect of UV-Radiation on the Packaging-Related Properties of Whey Protein Isolate Based Films and Coatings. Packag. Technol. Sci. 2015, 28, 883–899. [Google Scholar] [CrossRef]
- Michael, F.M.; Khalid, M.; Walvekar, R.; Siddiqui, H.; Balaji, A.B. Surface Modification Techniques of Biodegradable and Biocompatible Polymers. In Biodegradable and Biocompatible Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 33–54. ISBN 9780081009703. [Google Scholar]
- Romani, V.P.; Martins, V.G.; Goddard, J.M. Radical Scavenging Polyethylene Films as Antioxidant Active Packaging Materials. Food Control 2020, 109, 106946. [Google Scholar] [CrossRef]
- Goddard, J.M.; Talbert, J.N.; Hotchkiss, J.H. Covalent Attachment of Lactase to Low-Density Polyethylene Films. J. Food Sci. 2007, 72, E036–E041. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Kristinsson, H.G. ACE-Inhibitory Activity of Tilapia Protein Hydrolysates. Food Chem. 2009, 117, 582–588. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymatic Hydrolysis of Food Proteins; Elsevier Applied Science Publishing: London, UK, 1986. [Google Scholar]
- Kessler, F.; Kühn, S.; Radtke, C.; Weibel, D.E. Controlling the Surface Wettability of Poly(Sulfone) Films by UV-Assisted Treatment: Benefits in Relation to Plasma Treatment. Polym. Int. 2013, 62, 310–318. [Google Scholar] [CrossRef]
- Kessler, F.; Steffens, D.; Lando, G.A.; Pranke, P.; Weibel, D.E. Wettability and Cell Spreading Enhancement in Poly(Sulfone) and Polyurethane Surfaces by UV-Assisted Treatment for Tissue Engineering Purposes. Tissue Eng. Regen. Med. 2014, 11, 23–31. [Google Scholar] [CrossRef]
- D882-02; ASTM Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Material: Philadelphia, PA, USA, 2002.
- Silva Filipini, G.; Romani, V.P.; Guimarães Martins, V. Blending Collagen, Methylcellulose, and Whey Protein in Films as a Greener Alternative for Food Packaging: Physicochemical and Biodegradable Properties. Packag. Technol. Sci. 2021, 34, 91–103. [Google Scholar] [CrossRef]
- ASTM. ASTM E96: Standard Test Methods for Water Vapor Transmission of Materials. Annu. B. ASTM Stand. 2000, 4, 784–792. [Google Scholar]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the Incorporation of Antioxidants on Physicochemical and Antioxidant Properties of Wheat Starch-Chitosan Films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-Based Films Additivated with Curcuma Ethanol Extract: Antioxidant Activity and Physical Properties of Films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.S.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.M. Determination of Reducing Power and Metal Chelating Ability of Antioxidant Peptides: Revisited Methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- AOCS. AOCS Official Methods and Recommended Practices of the American Oil Chemists Society; AOCS Press: Champaign, IL, USA, 1998. [Google Scholar]
- Song, N.B.; Lee, J.H.; Al Mijan, M.; Song, K. Bin Development of a Chicken Feather Protein Film Containing Clove Oil and Its Application in Smoked Salmon Packaging. LWT Food Sci. Technol. 2014, 57, 453–460. [Google Scholar] [CrossRef]
- Gennadios, A.; Rhim, J.-W.; Handa, A.; Weller, C.L.; Hanna, M.A. Ultraviolet Radiation Affects Physical and Molecular Properties of Soy Protein Films. J. Food Sci. 1998, 63, 131–146. [Google Scholar] [CrossRef]
- Khan, B.; Khan Niazi, M.B.; Jahan, Z.; Farooq, W.; Naqvi, S.R.; Ali, M.; Ahmed, I.; Hussain, A. Effect of Ultra-Violet Cross-Linking on the Properties of Boric Acid and Glycerol Co-Plasticized Thermoplastic Starch Films. Food Packag. Shelf Life 2019, 19, 184–192. [Google Scholar] [CrossRef]
- Ustunol, Z.; Mert, B. Water Solubility, Mechanical, Barrier, and Thermal Properties of Cross-Linked Whey Protein Isolate-Based Films. J. Food Sci. 2006, 69, 129–133. [Google Scholar] [CrossRef]
- Díaz, O.; Candia, D.; Cobos, Á. Whey Protein Film Properties as Affected by Ultraviolet Treatment under Alkaline Conditions. Int. Dairy J. 2017, 73, 84–91. [Google Scholar] [CrossRef]
- Rhim, J.W.; Gennadios, A.; Fu, D.; Weller, C.L.; Hanna, M.A. Properties of Ultraviolet Irradiated Protein Films. LWT Food Sci. Technol. 1999, 32, 129–133. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Bourke, P.; Cullen, P.J. Zein Film: Effects of Dielectric Barrier Discharge Atmospheric Cold Plasma. J. Appl. Polym. Sci. 2014, 131, 9541–9546. [Google Scholar] [CrossRef]
- Detduangchan, N.; Wittaya, T. Effect of UV-Treatment on Properties of Biodegradable Film From Rice Starch. World Acad. Sci. Eng. Technol. 2011, 57, 464–469. [Google Scholar]
- Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Physical and Mechanical Properties of Chitosan Films as Affected by Drying Methods and Addition of Antimicrobial Agent. J. Food Eng. 2013, 119, 140–149. [Google Scholar] [CrossRef]
- Martin, I.; Goormaghtigh, E.; Ruysschaert, J.-M. Attenuated Total Reflection IR Spectroscopy as a Tool to Investigate the Orientation and Tertiary Structure Changes in Fusion Proteins. Biochim. Biophys. Acta Biomembr. 2003, 1614, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Lipid-Protein Interactions. In Lipid-Protein Interactions; Kleinschmidt, J.H., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 974, pp. 177–218. ISBN 978-1-62703-274-2. [Google Scholar]
- Rostami, A.H.; Motamedzadegan, A.; Hosseini, S.E.; Rezaei, M.; Kamali, A. Evaluation of Plasticizing and Antioxidant Properties of Silver Carp Protein Hydrolysates in Fish Gelatin Film. J. Aquat. Food Prod. Technol. 2017, 26, 457–467. [Google Scholar] [CrossRef]
- Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Antioxidant and Functional Properties of Gelatin Hydrolysates Obtained from Skin of Sole and Squid. Food Chem. 2009, 114, 976–983. [Google Scholar] [CrossRef]
- Halal, S.L.M.E.; Zavareze, E.D.R.; Da Rocha, M.; Pinto, V.Z.; Nunes, M.R.; Luvielmo, M.D.M.; Prentice, C. Films Based on Protein Isolated from Croaker (Micropogonias furnieri) and Palm Oil. J. Sci. Food Agric. 2016, 96, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Wiriyaphan, C.; Chitsomboon, B.; Yongsawadigul, J. Antioxidant Activity of Protein Hydrolysates Derived from Threadfin Bream Surimi Byproducts. Food Chem. 2012, 132, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Alhadhrami, A.S.; ALHashmi, D.S.H.; Jawad, M.; Dıblan, S.; Al Dawery, S.K.H.; Esatbeyoglu, T.; Anwer, M.K.; et al. Characterization of Biodegradable Films Based on Guar Gum and Calcium Caseinate Incorporated with Clary Sage Oil: Rheological, Physicochemical, Antioxidant, and Antimicrobial Properties. J. Agric. Food Res. 2024, 15, 100948. [Google Scholar] [CrossRef]
- Akhtar, H.M.S.; Zhao, Y.; Li, L.; Shi, Q. Novel Active Composite Films Based on Carboxymethyl Cellulose and Sodium Alginate Incorporated with Phycocyanin: Physico-Chemical, Microstructural and Antioxidant Properties. Food Hydrocoll. 2024, 147, 109440. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Saif Alrasbi, A.N.; Jawad, M.; Koca, E.; Aydemir, L.Y.; Alamoudi, J.A.; Almoshari, Y.; Mohan, S. Structural, Mechanical, Barrier and Antioxidant Properties of Pectin and Xanthan Gum Edible Films Loaded with Grapefruit Essential Oil. Heliyon 2024, 10, e25501. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Shan, M.; Zhang, L.; Chen, N.; Tie, H.; Xiao, Y.; Li, Z. Preparation of Gelatin/ĸ-Carrageenan Active Films through Procyanidins Crosslinking: Physicochemical, Structural, Antioxidant and Controlled Release Properties. Food Hydrocoll. 2024, 153, 110023. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Aurrekoetxea, G.P.; Angulo, I.; Paseiro-Losada, P.; Cruz, J.M. Development of New Active Packaging Films Coated with Natural Phenolic Compounds to Improve the Oxidative Stability of Beef. Meat Sci. 2014, 97, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Wickramarachchi, S.; Pal, K.; Sarkar, P. Gelatin/Chitosan-Lactate/Curcuma Hydroethanolic Extract-Based Antimicrobial Films: Preparation, Characterization, and Application on Chicken Meat. Food Hydrocoll. 2024, 154, 110075. [Google Scholar] [CrossRef]
- Insausti, K.; Beriain, M.J.; Purroy, A.; Alberti, P.; Gorraiz, C.; Alzueta, M.J. Shelf Life of Beef from Local Spanish Cattle Breeds Stored under Modified Atmosphere. Meat Sci. 2001, 57, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Alemán, A.; Pérez-Santín, E.; Bordenave-Juchereau, S.; Arnaudin, I.; Gómez-Guillén, M.C.; Montero, P. Squid Gelatin Hydrolysates with Antihypertensive, Anticancer and Antioxidant Activity. Food Res. Int. 2011, 44, 1044–1051. [Google Scholar] [CrossRef]



| Properties | No UV | UV 1 min | UV 5 min | UV 10 min |
|---|---|---|---|---|
| Thickness (mm) | 0.081 ± 0.02 a | 0.074 ± 0.03 a | 0.084 ± 0.06 a | 0.082 ± 0.04 a |
| Tensile strength (MPa) | 13.85 ± 0.05 b | 18.31 ± 0.96 a | 16.26 ± 0.64 ab | 17.24 ± 1.49 a |
| Elongation at break (%) | 35.4 ± 2.1 a | 11.0 ± 0.6 b | 5.0 ± 0.1 c | 5.2 ± 0.5 c |
| Water vapor permeability (g.mm/day.m2.kPa) | 7.47 ± 0.84 a | 7.23 ± 0.18 a | 7.37 ± 0.21 a | 6.61 ± 0.27 a |
| Solubility in water (%) | 20.8 ± 0.4 a | 21.6 ± 1.0 a | 24.0 ± 0.6 a | 22.1 ± 2.0 a |
| Total difference in color (ΔE*) | 5.44 ± 0.33 b | 5.46 ± 0.21 b | 6.57 ± 0.11 a | 7.04 ± 0.34 a |
| Opacity (%) | 10.7 ± 0.2 b | 11.3 ± 0.0 a | 11.5 ± 0.2 a | 11.2 ± 0.2 a |
| Film Sample | ABTS Assay (%) | Reducing Power (700 nm) |
|---|---|---|
| Control (no hydrolysates) | 64.8 ± 0.2 b | 0.257 ± 0.004 c |
| Protein hydrolysates in composition | 77.1 ± 0.5 a | 0.378 ± 0.011 a |
| Protein hydrolysates on the surface | 78.3 ± 0.6 a | 0.331 ± 0.005 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, V.P.; Martins, P.C.; da Rocha, M.; Bulhosa, M.C.S.; Kessler, F.; Martins, V.G. UV Radiation and Protein Hydrolysates in Bio-Based Films: Impacts on Properties and Italian Salami Preservation. Antioxidants 2024, 13, 517. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050517
Romani VP, Martins PC, da Rocha M, Bulhosa MCS, Kessler F, Martins VG. UV Radiation and Protein Hydrolysates in Bio-Based Films: Impacts on Properties and Italian Salami Preservation. Antioxidants. 2024; 13(5):517. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050517
Chicago/Turabian StyleRomani, Viviane Patrícia, Paola Chaves Martins, Meritaine da Rocha, Maria Carolina Salum Bulhosa, Felipe Kessler, and Vilásia Guimarães Martins. 2024. "UV Radiation and Protein Hydrolysates in Bio-Based Films: Impacts on Properties and Italian Salami Preservation" Antioxidants 13, no. 5: 517. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050517