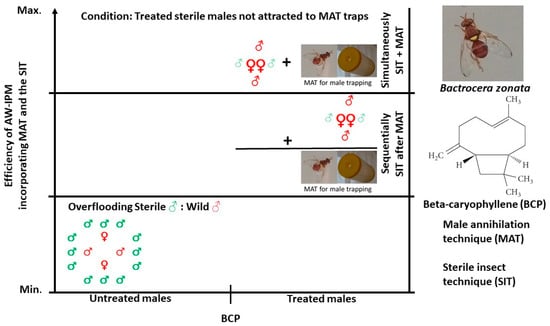
Consumption of β-Caryophyllene Increases the Mating Success of Bactrocera zonata Males (Diptera: Tephritidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Insects
2.2. ME Feeding
2.3. BCP Feeding
2.4. No-ME and No-BCP Treatment
2.5. Field Cages
2.6. Effect of ME and BCP Feeding on Male Mating Competitiveness
2.6.1. Experiment 1
2.6.2. Experiment 2
2.6.3. Experiment 3
2.6.4. Experiment 4
2.7. Effect of ME and BCP Feeding on Male Response to ME-Baited Traps
2.8. Data Analysis
3. Results
3.1. Mating Competitiveness
3.2. Male Response to ME-Baited Traps
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prokopy, R.J.; Vargas, R.I. Attraction of Ceratitis Capitata (Diptera: Tephritidae) flies to odor of coffee fruit. J. Chem. Ecol. 1996, 22, 807–820. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Moericke, V.; Bush, G.L. Attraction of apple maggot flies to odor of apples. Environ. Entomol. 1973, 2, 743–750. [Google Scholar] [CrossRef]
- Robacker, D.C. Specific hunger in Anastrepha ludens (Diptera: Tephritidae): Effects on attractiveness of proteinaceous and fruit-derived lures. Environ. Entomol. 1991, 20, 1680–1686. [Google Scholar] [CrossRef]
- Alyokhin, A.V.; Messing, R.H.; Duan, J.J. Visual and olfactory stimuli and fruit maturity affect trap captures of oriental fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 2000, 93, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.C. Notes and exhibitions. Proc. Hawaii. Entomol. Soc. 1965, 19, 23. [Google Scholar]
- Kawano, Y.; Mitchell, W.C.; Matsumoto, H. Identification of the male oriental fruit fly attractant in the golden shower blossom. J. Econ. Entomol. 1968, 61, 986–988. [Google Scholar] [CrossRef]
- Nishida, R.; Shelly, T.E.; Kaneshiro, K.Y. Acquisition of female-attracting fragrance by males of oriental fruit fly from a Hawaiian Lei Flower, Fagraea berteriana. J. Chem. Ecol. 1997, 23, 2275–2285. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Synomone or kairomone?—Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies. J. Chem. Ecol. 2005, 31, 497–507. [Google Scholar]
- Tan, K.H.; Nishida, R.; Toong, Y.C. Floral synomone of a wild orchid, Bulbophyllum cheiri, lures Bactrocera fruit flies for pollination. J. Chem. Ecol. 2002, 28, 1161–1172. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12, 56. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H.; Fukami, H. Cis-3, 4-Dimethoxycinnamyl alcohol from the rectal glands of male oriental fruit fly, Dacus dorsalis. Chem. Express 1988, 3, 207–210. [Google Scholar]
- Tan, K.H.; Nishida, R. Sex pheromone and mating competition after methyl eugenol consumption in the Bactrocera dorsalis complex. In Fruit Fly Pests: A World Assessment of their Biology and Management; Mcpheron, B.A., Steck, G.J., Eds.; St. Lucie Press: Delray Beach, FL, USA, 1996; pp. 147–153. [Google Scholar]
- Shelly, T.E.; Dewire, M. Chemically mediated mating success in male oriental fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1994, 87, 375–382. [Google Scholar] [CrossRef]
- Hee, A.K.W.; Tan, K.H. Attraction of female and male Bactrocera papayae to conspecific males fed with methyl eugenol and attraction of females to male sex pheromone components. J. Chem. Ecol. 1998, 24, 753–764. [Google Scholar] [CrossRef]
- Orankanok, W.; Chinvinijkul, S.; Sawatwangkhoung, A.; Pinkaew, S.; Orankanok, S. Methyl eugenol and pre-release diet improve mating performance of young Bactrocera dorsalis and Bactrocera correcta males. J. Appl. Entomol. 2013, 137, 200–209. [Google Scholar] [CrossRef]
- Haq, I.; Cáceres, C.; Meza, J.S.; Hendrichs, J.; Vreysen, M.J.B. Different methods of methyl eugenol application enhance the mating success of male oriental fruit fly (Dipera: Tephritidae). Sci. Rep. 2018, 8, 6033. [Google Scholar] [CrossRef]
- Wee, S.L.; Tan, K.H.; Nishida, R. Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae. J. Chem. Ecol. 2007, 33, 1272–1282. [Google Scholar] [CrossRef]
- Haq, I.; Vreysen, M.J.B.; Cáceres, C.; Shelly, T.E.; Hendrichs, J. Methyl eugenol aromatherapy enhance the mating competitiveness of male Bactrocera carambolae Drew & Hancock (Diptera: Tephritidae). J. Insect Physiol. 2014, 68, 1–6. [Google Scholar] [PubMed]
- Wee, S.L.; Abdul Munir, M.Z.; Hee, A.K.W. Attraction and consumption of methyl eugenol by male Bactrocera umbrosa Fabricius (Diptera: Tephritidae) promotes conspecific sexual communication and mating performance. Bull. Entomol. Res. 2018, 108, 116–124. [Google Scholar] [CrossRef]
- Wee, S.L.; Hee, A.K.W.; Tan, K.H. Comparative sensitivity to and consumption of methyl eugenol in three Bactrocera dorsalis (Diptera: Tephritidae) complex sibling species. Chemoecology 2002, 12, 193–197. [Google Scholar] [CrossRef]
- Haq, I.; Vreysen, M.J.B.; Schutze, M.; Hendrichs, J.; Shelly, T. Effects of methyl eugenol feeding on mating compatibility of Asian population of Bactrocera dorsalis (Diptera: Tephritidae) with African population and with B. carambolae. J. Econ. Entomol. 2016, 109, 148–153. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 2002. [Google Scholar]
- Quilici, S.; Donner, P. Analysis of exotic fruit fly trapping networks. EPPO Bull. 2012, 42, 102–108. [Google Scholar] [CrossRef]
- Suckling, D.M.; Kean, J.M.; Stringer, L.D.; Cáceres, C.E.; Hendrichs, J.; Reyes-Flores, J.; Dominiak, B.C. Eradication of tephritid fruit fly pest populations: Outcomes and prospects. Pest Manag. Sci. 2016, 72, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Leblanc, L.; Piñero, J.C.; Hoffman, K.M. Male annihilation, past, present, and future. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T.E., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R.I., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 493–511. [Google Scholar]
- Knipling, E.F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Shelly, T.E.; Edu, J.; McInnis, D. Pre-release consumption of methyl eugenol increases the mating competitiveness of sterile males of the oriental fruit fly, Bactrocera dorsalis, in large field enclosures. J. Insect Sci. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- EPPO (European and Mediterranean Plant Protection Organization). EPPO Datasheet: Bactrocera zonata. EPPO Datasheets on Pests Recommended for Regulation. 2023. Available online: https://gd.eppo.int (accessed on 28 April 2021).
- Khan, M.M.; Sha, S.W.H.; Akhter, I.; Malik, H. Integrated pest management of fruit flies in guava orchards. J. Entomol. Zool. Stud. 2017, 5, 135–138. [Google Scholar]
- Abubakar, M.; Ali, H.; Shad, S.A.; Anees, M.; Binyameen, M. Trichlorfon resistance: Its stability and impacts on biological parameters of Bactrocera zonata (Diptera: Tephritidae). Appl. Entomol. Zool. 2021, 56, 473–482. [Google Scholar] [CrossRef]
- Hasnain, M.; Saeed, S.; Ullah, U.N.; Ullah, S.; Zaka, S.M. Synergist response of the peach fruit fly, Bactrocera zonata (Saunders) to some ammonium based proteinaceous food bait attractants. BMC Zool. 2023, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- EPPO (European and Mediterranean Plant Protection Organization). Bactrocera zonata: Procedure for Official Control. EPPO Bull. 2010, 40, 390–395. [Google Scholar] [CrossRef]
- Ghanim, N.; Moustafa, S.; El-Metwally, M.; Afia, Y.; Salman, M.; Mostafa, M. Efficiency of some insecticides in male annihilation technique of peach fruit fly, Bactrocera zonata (Saunders) under Egyptian conditions. Egypt. Acad. J. Biol. Sci. 2010, 2, 13–19. [Google Scholar] [CrossRef]
- Sookar, P.; Alleck, M.; Ahseek, N.; Permalloo, S.; Bhagwant, S.; Chang, C.L. Artificial rearing of the peach fruit fly Bactrocera zonata (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2014, 34, S99–S107. [Google Scholar] [CrossRef]
- Ndzana, A.R.; Turlings, T.; Woin, N.; Quilici, S. Factors influencing the mating success of Bactrocera zonata (Saunders) (Diptera: Tephritidae) males in an SIT programme. In Proceedings of the 3rd International Symposium of Workers of Europe, Africa and the Middle East (TEAM), Stellenbosch, South Africa, 11 April 2016. [Google Scholar]
- Patel, N.A.; Facknath, S.; Sookar, P. Development of a waste brewery yeast larval diet for rearing Bactrocera zonata for use in SIT. Phytoparasitica 2023, 51, 285–303. [Google Scholar] [CrossRef]
- Rasool, A.; Munis, M.F.H.; Shah, S.H.; Fatima, S.; Irshad, A.; Haq, I. Age-dependent effect of methyl eugenol on male mating success of the peach fruit fly, Bactrocera zonata. Entomol. Exp. Appl. 2023, 171, 838–845. [Google Scholar] [CrossRef]
- Maffei, M.E. Plant natural sources of the endocannabinoid (E)-β-Caryophyllene: A systematic quantitative analysis of published literature. Int. J. Mol. Sci. 2020, 21, 6540. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Methyl Eugenol (CAS NO. 93-15-2) in F344/N Rats and B6C3F1 Mice (Gavage Studies); National Toxicology Program Technical Report Series, No. 491; National Toxicology Program: Research Triangle Park, NC, USA, 2000; pp. 1–412.
- National Toxicology Program. Report on Carcinogens, 10th ed.; National Toxicology Program: Research Triangle Park, NC, USA, 2002.
- Barclay, H.J.; Hendrichs, J. Modeling Trapping of Fruit Flies for Detection, Suppression, or Eradication. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies; Shelly, T.E., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R.I., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 379–420. [Google Scholar]
- FAO/IAEA (Food and Agriculture Organization/International Atomic Energy Agency). Trapping Guidelines for Area-Wide Fruit Fly Programmes, 2nd ed.; Enkerlin, W.R., Reyes-Flores, J., Eds.; FAO: Rome, Italy, 2018. [Google Scholar]
- Wee, S.L.; Chinvinijkul, S.; Tan, K.H.; Nishida, R. A new and highly effective male lure for the guava fruit fly Bactrocera correcta. J. Pest Sci. 2018, 91, 691–698. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H.; Serit, M.; Lajis, N.H.; Sukari, A.M.; Takahashi, S.; Fukami, H. Accumulation of phenylpropanoids in the rectal glands of males of the oriental fruit fly, Dacus dorsalis. Experientia 1988, 44, 534–536. [Google Scholar] [CrossRef]
- Tokushima, I.; Orankanok, W.; Tan, K.H.; Ono, H.; Nishida, R. Accumulation of phenylpropanoid and sesquiterpenoid volatiles in male rectal pheromonal glands of the guava fruit fly, Bactrocera correcta. J. Chem. Ecol. 2010, 36, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Tan, K.H. Automated tephritid fruit fly semiochemical mass feeding structure: Design, construction and testing. J. Appl. Entomol. 2013, 137, 217–229. [Google Scholar] [CrossRef]
- Shelly, T.E.; Rendon, P.; Moscoso, F.; Menendez, R. Testing the efficacy of aromatherapy at the world’s largest eclosion facility for sterile males of the Mediterranean fruit fly (Diptera: Tephritidae). Proc. Hawaii. Entomol. Soc. 2010, 42, 33–40. [Google Scholar]
- Rasool, A.; Fatima, S.; Shah, S.H.; Munis, M.F.H.; Irshad, A.; Shelly, T.E.; Haq, I. Methyl eugenol aromatherapy: A delivery system facilitating the simultaneous application of male annihilation and sterile insect technique against the peach fruit fly. Pest Manag. Sci. 2024, 3, 1465–1473. [Google Scholar] [CrossRef]
- Zingore, K.M.; Sithole, G.; Abdel-Rahman, E.M.; Mohamed, S.A.; Ekesi, S.; Tanga, C.M.; Mahmoud, M.E.E. Global risk of invasion by Bactrocera zonata: Implications on horticultural crop production under changing climatic conditions. PLoS ONE 2020, 15, e0243047. [Google Scholar] [CrossRef]

| Experiment | Competing Males | Matings | Test Statistic | p |
|---|---|---|---|---|
| 1 | ME-fed | 11.9 (1.1) | t = 7.31 | <0.001 |
| Untreated | 7.0 (1.5) | |||
| 2 | BCP-fed | 9.9 (2.2) | t = 2.44 | 0.03 |
| Untreated | 7.5 (1.7) | |||
| 3 | ME-fed | 8.9 (1.9) | T = 68.0 | 1.0 |
| BCP-fed | 9.0 (1.4) | |||
| 4 | ME-fed | 6.0 (2.4) | F = 4.65 | 0.02 |
| BCP-fed | 8.6 (3.0) | |||
| Untreated | 5.1 (1.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ul Haq, I.; Fatima, S.; Rasool, A.; Shelly, T.E. Consumption of β-Caryophyllene Increases the Mating Success of Bactrocera zonata Males (Diptera: Tephritidae). Insects 2024, 15, 310. https://0-doi-org.brum.beds.ac.uk/10.3390/insects15050310
ul Haq I, Fatima S, Rasool A, Shelly TE. Consumption of β-Caryophyllene Increases the Mating Success of Bactrocera zonata Males (Diptera: Tephritidae). Insects. 2024; 15(5):310. https://0-doi-org.brum.beds.ac.uk/10.3390/insects15050310
Chicago/Turabian Styleul Haq, Ihsan, Sehar Fatima, Awais Rasool, and Todd E. Shelly. 2024. "Consumption of β-Caryophyllene Increases the Mating Success of Bactrocera zonata Males (Diptera: Tephritidae)" Insects 15, no. 5: 310. https://0-doi-org.brum.beds.ac.uk/10.3390/insects15050310