Electrochemical Behavior of Natural Manganese Oxides: Transforming Mining Waste into Energy Storage Materials
Abstract
:1. Introduction
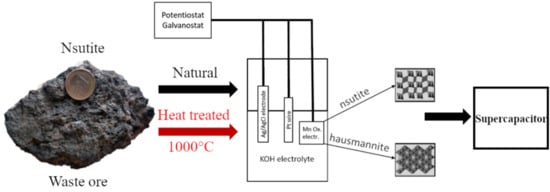
2. Materials and Methods
2.1. Sample Selection
2.2. Mineralogical Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. X-ray Diffraction Analysis
- (a)
- The more or less random stacking of the ramsdellite and pyrolusite structures;
- (b)
- The micro-twinning of the ramsdellite lattice in 021/061 planes.
- A (0,0) is the ideal-defectless ramsdellite;
- B (1,0) represents the pyrolusite;
- C (0,100) describes the fully twinned structure of ramsdellite;
- D (100,0) represents the fully twinned pyrolusite.
3.2. Transmission Electron Microscope Analysis
3.3. Electrochemical Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, S.K. Diversity in the Family of Manganese Oxides at the Nanoscale: From Fundamentals to Applications. ACS Omega 2020, 5, 25493–25504. [Google Scholar] [CrossRef]
- Yang, X.; Peng, Q.; Liu, L.; Tan, W.; Qiu, G.; Liu, C.; Dang, Z. Synergistic Adsorption of Cd(II) and As(V) on Birnessite under Electrochemical Control. Chemosphere 2020, 247, 125822. [Google Scholar] [CrossRef]
- Rout, K.; Mohapatra, M.; Mohapatra, B.K.; Anand, S. Pb(II), Cd(II) and Zn(II) Adsorption on Low Grade Manganese Ore. Int. J. Eng. Sci. Technol. 2009, 1, 106–122. [Google Scholar] [CrossRef]
- Radinger, H.; Connor, P.; Stark, R.; Jaegermann, W.; Kaiser, B. Manganese Oxide as an Inorganic Catalyst for the Oxygen Evolution Reaction Studied by X-ray Photoelectron and Operando Raman Spectroscopy. ChemCatChem 2021, 13, 1175–1185. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese Oxides for Lithium Batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef]
- Post, J.E. Manganese Oxide Minerals: Crystal Structures and Economic and Environmental Significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Post, J.E.; McKeown, D.A.; Heaney, P.J. Raman Spectroscopy Study of Manganese Oxides: Tunnel Structures. Am. Miner. 2020, 105, 1175–1190. [Google Scholar] [CrossRef]
- Post, J.E.; McKeown, D.A.; Heaney, P.J. Raman Spectroscopy Study of Manganese Oxides: Layer Structures. Am. Miner. 2021, 106, 351–366. [Google Scholar] [CrossRef]
- Kondo, T.; Matsushima, Y.; Matsuda, K.; Unuma, H. Electrochemical Properties of δ- and γ-MnO2 Thin Films Deposited by a Chemical Bath Technique. J. Mater. Sci. Mater. Electron. 2016, 27, 8001–8005. [Google Scholar] [CrossRef]
- Magnard, N.P.L.; Anker, A.S.; Aalling-Frederiksen, O.; Kirsch, A.; Jensen, K.M. Characterisation of Intergrowth in Metal Oxide Materials Using Structure-Mining: The Case of γ-MnO2. Dalton Trans. 2022, 51, 17150–17161. [Google Scholar] [CrossRef]
- de Wolff, P.M. Interpretation of Some γ-MnO2 Diffraction Patterns. Acta Crystallogr. 1959, 12, 341–345. [Google Scholar] [CrossRef]
- Chabre, Y.; Pannetier, J. Structural and Electrochemical Properties of the Proton/γ-MnO2 System. Prog. Solid State Chem. 1995, 23, 1–130. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Lv, T.-T.; Wang, H.; Guo, X.-T.; Liu, C.-S.; Pang, H. Nsutite-type VO2 microcrystals as highly durable cathode materials for aqueous zinc-Ion batteries. Chem. Eng. J. 2021, 417, 128408. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef]
- Sofianska, E.; Michailidis, K. Assessment of heavy metals contamination and potential ecological risk in soils affected by a former Mn mining activity, Drama District, Northern Greece. Soil Sediment Contam. Int. J. 2016, 25, 296–312. [Google Scholar] [CrossRef]
- Gomes-Pimentel, M.; da Silva, M.R.C.; Viveiros, D.d.C.S.; Picanço, M.S. Manganese Mining Waste as a Novel Supplementary Material in Portland Cement. Mater. Lett. 2022, 309, 131459. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Araújo, C.F.; dos Santos, N.R.; Bandeira, M.J.; Anjos, A.L.S.; Carvalho, C.F.; Lima, C.S.; Abreu, J.N.S.; Mergler, D.; Menezes-Filho, J.A. Airborne Manganese Exposure and Neurobehavior in School-Aged Children Living near a Ferro-Manganese Alloy Plant. Environ. Res. 2018, 167, 66–77. [Google Scholar] [CrossRef]
- Rivera-Becerril, F.; Juárez-Vázquez, L.V.; Hernández-Cervantes, S.C.; Acevedo-Sandoval, O.A.; Vela-Correa, G.; Cruz-Chávez, E.; Moreno-Espíndola, I.P.; Esquivel-Herrera, A.; de León-González, F. Impacts of Manganese Mining Activity on the Environment: Interactions Among Soil, Plants, and Arbuscular Mycorrhiza. Arch. Environ. Contam. Toxicol. 2013, 64, 219–227. [Google Scholar] [CrossRef]
- Troy, P.J.; Wiltshire, J.C. Manganese tailings: Useful properties suggest a potential for gas absorbent and ceramic materials. Mar. Georesources Geotechnol. 1998, 16, 273–281. [Google Scholar] [CrossRef]
- Santos, O.d.S.H.; Carvalho, C.d.F.; da Silva, G.A.; dos Santos, C.G. Manganese ore tailing: Optimization of acid leaching conditions and recovery of soluble manganese. J. Environ. Manag. 2015, 147, 314–320. [Google Scholar] [CrossRef]
- Yao, J.; Yu, T.; Huang, Q.; Li, Y.; Huang, B.; Yang, J. Preparation of Mn2O3/Fe2O3 composite cathode material for zinc ion batteries from the reduction leaching solution of manganese ore tailing. J. Ind. Eng. Chem. 2024, in press. [CrossRef]
- Michailidis, K.M.; Nicholson, K.; Nimfopoulos, M.K.; Pattrick, R.A.D. An EPMA and SEM Study of the Mn-Oxide Mineralization of Kato Nevrokopi, Macedonia, Northern Greece: Controls on Formation of the Mn4+ Oxides. Geol. Soc. Lond. Spéc. Publ. 1997, 119, 265–280. [Google Scholar] [CrossRef]
- Nimfopoulos, M. Manganese Mineralization near Kato Nevrokopi, Drama, Greece. Ph.D. Thesis, Geology Department, University of Manchester, Manchester, UK, 1988. [Google Scholar]
- Stötzel, C.; Müller, F.; Reinert, F.; Niederdraenk, F.; Barralet, J.; Gbureck, U. Ion Adsorption Behaviour of Hydroxyapatite with Different Crystallinities. Colloids Surfaces B Biointerfaces 2009, 74, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. B 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Hossain, S.; Mahmud, M.; Bin Mobarak, M.; Sultana, S.; Shaikh, A.A.; Ahmed, S. New Analytical Models for Precise Calculation of Crystallite Size: Application to Synthetic Hydroxyapatite and Natural Eggshell Crystalline Materials. Chem. Pap. 2022, 76, 7245–7251. [Google Scholar] [CrossRef]
- Kulkarni, S.; Puthusseri, D.; Thakur, S.; Banpurkar, A.; Patil, S. Hausmannite Manganese Oxide Cathodes for Supercapacitors: Surface Wettability and Electrochemical Properties. Electrochim. Acta 2017, 231, 460–467. [Google Scholar] [CrossRef]
- McMurdie, H.F.; Golovato, E. Study of the Modifications of Manganese Dioxide. J. Res. Natl. Bur. Stand. 1948, 41, 589. [Google Scholar] [CrossRef]
- Bish, D.L.; Post, J.E. Thermal Behavior of Complex, Tunnel-Structure Manganese Oxides. Am. Mineral. 1989, 74, 177–186. [Google Scholar]
- Stoševski, I.; Bonakdarpour, A.; Fang, B.; Voon, S.T.; Wilkinson, D.P. Hausmannite Mn3O4 as a Positive Active Electrode Material for Rechargeable Aqueous Mn-Oxide/Zn Batteries. Int. J. Energy Res. 2021, 45, 220–230. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Li, C.; Yan, C.; Lee, P.S.; Ma, J. High–Rate Electrochemical Capacitors from Highly Graphitic Carbon–Tipped Manganese Oxide/Mesoporous Carbon/Manganese Oxide Hybrid Nanowires. Energy Environ. Sci. 2011, 4, 1813–1819. [Google Scholar] [CrossRef]
- Nguyen, T.; Boudard, M.; Carmezim, M.J.; Montemor, M.F. Layered Ni(OH)2-Co(OH)2 Films Prepared by Electrodeposition as Charge Storage Electrodes for Hybrid Supercapacitors. Sci. Rep. 2017, 7, 39980. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for Electrochemical Capacitors and Related Devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Lee, S.C.; Jung, W.Y. Analogical Understanding of the Ragone Plot and a New Categorization of Energy Devices. Energy Procedia 2016, 88, 526–530. [Google Scholar] [CrossRef]
- Singhal, R.; Lembeck, J.; LeMaire, P.C.K., III; Pereira, D.; LeMaire, P. Physical and Electrochemical Characterization of Nsutite. Am. J. Anal. Chem. 2021, 12, 440–445. [Google Scholar] [CrossRef]
- Faulring, G.M. A study of Cuban Todorokite. Adv. X-ray Anal. 1961, 5, 117–126. [Google Scholar] [CrossRef]
- Graf, D.L. Crystallographic tables for the rhombohedral carbonates. Am. Mineral. 1961, 46, 1283–1316. [Google Scholar]
- Soulamidis, G.; Stouraiti, C.; Kourmousi, M.; Tzevelekidis, P.; Charalampous, E.; Mitsopoulou, C.A. Structural and Electrochemical Characterization of Natural Manganese Oxides for Energy Storage Applications. Mater. Proc. 2023, 15, 64. [Google Scholar] [CrossRef]
- Malinger, K.A.; Laubernds, K.; Son, Y.-C.; Suib, S.L. Effects of Microwave Processing on Chemical, Physical, and Catalytic Properties of Todorokite-Type Manganese Oxide. Chem. Mater. 2004, 16, 4296–4303. [Google Scholar] [CrossRef]
- de Almeida, W.L.; Freisleben, L.C.; Brambilla, B.C.; Isoppo, V.G.; Rodembusch, F.S.; de Sousa, V.C. Influence of Starch Used in the Sol-Gel Synthesis of ZnO Nanopowders. J. Nanopart. Res. 2023, 25, 75. [Google Scholar] [CrossRef]
- Stouraiti, C.; Lozios, S.; Soukis, K.; Mavrogonatos, C.; Tsikos, H.; Voudouris, P.; Wang, H.; Zamparas, C.; Kollias, K. Manganese Metallogenesis in the Hellenic Arc: Case Studies from a Triassic Rift-Related Volcaniclastic Succession of the Cycladic Blueschist Unit, Greece. Ore Geol. Rev. 2022, 142, 104694. [Google Scholar] [CrossRef]
- Gowda, C.C.; Mathur, A.; Parui, A.; Kumbhakar, P.; Pandey, P.; Sharma, S.; Chandra, A.; Singh, A.K.; Halder, A.; Tiwary, C.S. Understanding the Electrocatalysis OER and ORR Activity of Ultrathin Spinel Mn3O4. J. Ind. Eng. Chem. 2022, 113, 153–160. [Google Scholar] [CrossRef]
- Chigane, M.; Ishikawa, M. Manganese Oxide Thin Film Preparation by Potentiostatic Electrolyses and Electrochromism. J. Electrochem. Soc. 2000, 147, 2246. [Google Scholar] [CrossRef]
- Verma, A.; Jha, A.; Basu, S. Manganese Dioxide as a Cathode Catalyst for a Direct Alcohol or Sodium Borohydride Fuel Cell with a Flowing Alkaline Electrolyte. J. Power Sources 2005, 141, 30–34. [Google Scholar] [CrossRef]
- Rus, E.D.; Moon, G.D.; Bai, J.; Steingart, D.A.; Erdonmez, C.K. Electrochemical Behavior of Electrolytic Manganese Dioxide in Aqueous KOH and LiOH Solutions: A Comparative Study. J. Electrochem. Soc. 2015, 163, A356. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Liu, L.; Manasa, P.; Kang, L.; Ran, F. Vanadium Nitride for Aqueous Supercapacitors: A Topic Review. J. Mater. Chem. A 2020, 8, 8218–8233. [Google Scholar] [CrossRef]
- Kavitha, E.; Meiyazhagan, S.; Yugeswaran, S.; Balraju, P.; Suresh, K. Electrochemical Prospects and Potential of Hausmannite Mn3O4 Nanoparticles Synthesized through Microplasma Discharge for Supercapacitor Applications. Int. J. Energy Res. 2021, 45, 7038–7056. [Google Scholar] [CrossRef]
- Taguchi, A.; Inoue, S.; Akamaru, S.; Hara, M.; Watanabe, K.; Abe, T. Phase transition and electrochemical capacitance of mechanically treated manganese oxides. J. Alloys Compd. 2006, 414, 137–141. [Google Scholar] [CrossRef]








| Nsutite [13] | Todorokite [37] | Calcite [38] | |||
|---|---|---|---|---|---|
| 2θ | hkl | 2θ | hkl | 2θ | hkl |
| 22.14 | 110 | 9.23 | 100 | 23.1 | 012 |
| 34.75 | 130 | 12.2 | 110 | 36 | 110 |
| 36.86 | 021 | 18.2 | 200 | 39.4 | 113 |
| 38.8 | 040 | 18.55 | 120 | 43.16 | 202 |
| 40.36 | 200 | 26.21 | 300 | 47.13 | 024 |
| 42.26 | 121 | 37.52 | 201 | 47.51 | 018 |
| 43.3 | 140 | 41.23 | 031 | 48.51 | 116 |
| 55.95 | 221 | 49.86 | 440 | ||
| 56.66 | 240 | 60.28 | 521 | ||
| Miller Indices | Ideal Ramsdellite | MXL3 | |||||
|---|---|---|---|---|---|---|---|
| h | k | l | 2θ (CuKa) | 2θ (CuKa) Measured | Δ2θ Ideal | Micro-Twinning | De Wolff |
| 1 | 1 | 0 | 21.8 | 22.14 | 0.34 | − | + |
| 1 | 3 | 0 | 35.1 | 34.75 | −0.35 | + | - |
| 0 | 2 | 1 | 36.8 | 36.86 | 0.06 | + | |
| 1 | 1 | 1 | 38.4 | - | − | − | |
| 0 | 4 | 0 | 38.8 | 38.4 | −0.4 | − | |
| 2 | 0 | 0 | 39.7 | 40.36 | 0.66 | ||
| 1 | 2 | 1 | 42 | 42.26 | 0.26 | + | |
| 1 | 4 | 0 | 43.8 | 43.3 | −0.5 | − | |
| 1 | 3 | 1 | 47.7 | 47.933 | 0.233 | - | + |
| 2 | 2 | 1 | 55.3 | 55.95 | 0.65 | + | |
| 2 | 4 | 0 | 56.8 | 56.66 | −0.14 | − | |
| 2 | 3 | 1 | 60 | - | - | − | + |
| 1 | 5 | 1 | 63.1 | 62.59 | −0.51 | + | − |
| 0 | 0 | 2 | 65 | 66.035 | 1.035 | + | |
| 0 | 6 | 1 | 69 | 68.09 | −0.91 | − | |
| 3 | 3 | 0 | 69.2 | 68.73 | −0.47 | + | − |
| 1 | 1 | 2 | 69.5 | 69.76 | 0.26 | − | + |
| 3 | 0 | 1 | 70.4 | - | − | ||
| 3 | 1 | 1 | 71.2 | - | − | − | |
| Sample Name | Mineral Phase | D-Crystallite Size (nm) |
|---|---|---|
| MXL1 | Todorokite | 25 |
| MXL3 | Nsutite (γ-MnO2) | 11 |
| d Spacing Calculated from SAED (nm) | d Spacing Calculated from pXRD (nm) | Hkl Lattices | 2theta Peak (pXRD) | |
|---|---|---|---|---|
| 1 | 0.2396 | 0.2336 | 0 2 1 | 36.89 |
| 2 | 0.2424 | 0.2434 | 0 4 0 | 38.4 |
| 3 | 0.1385 | 0.1375 | 0 0 2 | 66.035 |
| 4 | 0.1404 | 0.1413 | 0 6 1 | 68.09 |
| Electrodes | Current Density (A/g) | Specific Capacitance (F/g) | Energy Density (Wh/Kg) | Power Density (W/Kg) |
|---|---|---|---|---|
| MXL3 | 0.043 | 92.55 | 0.04 | 1.27 |
| 0.43 | 434.8 | 2.79 | 46.77 | |
| 43 | 17.39 | 60.38 | 107,684 | |
| MXL3HT | 0.167 | 191 | 0.45 | 10.82 |
| 1.67 | 667.3 | 5.93 | 213.78 | |
| 167 | 38.65 | 234.5 | 551,765 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soulamidis, G.; Kourmousi, M.; Mitsopoulou, C.A.; Stouraiti, C. Electrochemical Behavior of Natural Manganese Oxides: Transforming Mining Waste into Energy Storage Materials. Minerals 2024, 14, 455. https://0-doi-org.brum.beds.ac.uk/10.3390/min14050455
Soulamidis G, Kourmousi M, Mitsopoulou CA, Stouraiti C. Electrochemical Behavior of Natural Manganese Oxides: Transforming Mining Waste into Energy Storage Materials. Minerals. 2024; 14(5):455. https://0-doi-org.brum.beds.ac.uk/10.3390/min14050455
Chicago/Turabian StyleSoulamidis, George, Maria Kourmousi, Christiana A. Mitsopoulou, and Christina Stouraiti. 2024. "Electrochemical Behavior of Natural Manganese Oxides: Transforming Mining Waste into Energy Storage Materials" Minerals 14, no. 5: 455. https://0-doi-org.brum.beds.ac.uk/10.3390/min14050455