Assessing the Interactions between Zinc and Vitamin A on Intestinal Functionality, Morphology, and the Microbiome In Vivo (Gallus gallus)
Abstract
:1. Introduction
2. Materials and Methods
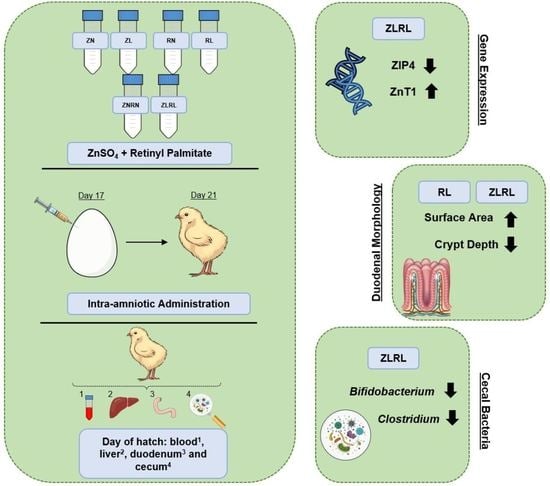
2.1. Zinc and Vitamin A Preparation
2.2. Animals and Design
2.3. Hepatic Retinol Content Quantification
2.4. Plasma Zinc Content Analysis
2.5. Total RNA Extraction
2.6. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
2.7. Microbial Samples and Intestinal Contents DNA Isolation
2.8. Primer Design and PCR Amplification of Bacterial 16S rDNA
2.9. Histomorphological Examination
2.10. Statistical Analysis
3. Results
3.1. Plasma Zinc and Liver Retinol Content
3.2. Gene Expression of Duodenal and Hepatic Zinc-Relevant Metabolism Proteins
3.3. Gene Expression of Duodenal and Hepatic Retinoid-Related Metabolism Proteins
3.4. Gene Expression of Duodenal Inflammatory-Related Proteins
3.5. Gene Expression of Duodenal Functionality-Relevant Proteins
3.6. Duodenal Morphology
3.7. Cecal Bacterial Abundance of Select Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Zinc and Vitamin A Preparation
References
- Stevens, G.A.; Beal, T.; Mbuya, M.N.N.; Luo, H.; Neufeld, L.M.; Addo, O.Y.; Adu-Afarwuah, S.; Alayón, S.; Bhutta, Z.; Brown, K.H.; et al. Micronutrient Deficiencies among Preschool-Aged Children and Women of Reproductive Age Worldwide: A Pooled Analysis of Individual-Level Data from Population-Representative Surveys. Lancet Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef]
- Wrottesley, S.V.; Mates, E.; Brennan, E.; Bijalwan, V.; Menezes, R.; Ray, S.; Ali, Z.; Yarparvar, A.; Sharma, D.; Lelijveld, N. Nutritional Status of School-Age Children and Adolescents in Low- and Middle-Income Countries across Seven Global Regions: A Synthesis of Scoping Reviews. Public Health Nutr. 2023, 26, 63–95. [Google Scholar] [CrossRef] [PubMed]
- Glutsch, V.; Hamm, H.; Goebeler, M. Zinc and Skin: An Update. JDDG J. Der Dtsch. Dermatol. Ges. 2019, 17, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessels, I.; Fischer, J.; Rink, L. Annual Review of Nutrition: Dietary and Physiological Effects of Zinc on the Immune System. Annu. Rev. Nutr. 2021, 41, 133–175. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [Green Version]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [Green Version]
- Maret, W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Wahed, M.A.; Fuchs, G.J.; Baqui, A.H.; Alvarez, J.O. Synergistic Effect of Zinc and Vitamin A on the Biochemical Indexes of Vitamin A Nutrition in Children. Am. J. Clin. Nutr. 2002, 75, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sklan, D.; Halevy, O.; Donoghue, S. The Effect of Different Dietary Levels of Vitamin A on Metabolism of Copper Iron and Zinc in the Chick. Int. J. Vitam. Nutr. Res. 1987, 57, 11–18. [Google Scholar]
- Christian, P.; West Jr., K.P. Interactions between Zinc and Vitamin A an Update. Am. J. Clin. Nutr. 1998, 68, 435S–441S. [Google Scholar] [CrossRef] [Green Version]
- Boron, B.; Hupert, J.; Barch, D.; Fox, C.; Friedman, H.; Layden, T.; Mobarhan, S. Effect of Zinc Deficiency on Hepatic Enzymes Regulating Vitamin A Status. Am. Inst. Nutr. 1987, 118, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Cvekl, A.; Wang, W.L. Retinoic Acid Signaling in Mammalian Eye Development. Exp. Eye Res. 2009, 89, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, R.; Peto, T.; Lengyel, I.; Emri, E. Zinc Nutrition and Inflammation in the Aging Retina. Mol. Nutr. Food Res. 2019, 63, 1801049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecil Smith, J.; McDaniel, E.G.; Fan, F.F.; Halsted, J.A. Zinc: A Trace Element Essential in Vitamin A Metabolism. Science 1973, 181, 954–955. [Google Scholar] [CrossRef]
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005 WHO Global Database on Vitamin A Deficiency; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Amimo, J.O.; Michael, H.; Chepngeno, J.; Raev, S.A.; Saif, L.J.; Vlasova, A.N. Immune Impairment Associated with Vitamin A Deficiency: Insights from Clinical Studies and Animal Model Research. Nutrients 2022, 14, 5038. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Thirunavukarasu, A.J.; Ross, A.C.; Gilbert, R.M. Vitamin A, Systemic T-Cells, and the Eye: Focus on Degenerative Retinal Disease. Front. Nutr. 2022, 9, 914457. [Google Scholar] [CrossRef]
- Reed, S.; Knez, M.; Uzan, A.; Stangoulis, J.C.R.; Glahn, R.P.; Koren, O.; Tako, E. Alterations in the Gut (Gallus gallus) Microbiota Following the Consumption of Zinc Biofortified Wheat (Triticum aestivum)-Based Diet. J. Agric. Food Chem. 2018, 66, 6291–6299. [Google Scholar] [CrossRef]
- Himoto, T.; Masaki, T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients 2018, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Imdad, A.; Mayo-Wilson, E.; Herzer, K.; Bhutta, Z.A. Vitamin A Supplementation for Preventing Morbidity and Mortality in Children from Six Months to Five Years of Age. Cochrane Database Syst. Rev. 2017, 3, CD00852. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009; ISBN 9789241563871. [Google Scholar]
- Kartasurya, M.I.; Marks, G.C.; Ahmed, F.; Subagio, H.W.; Rahfiludin, M.Z. Effect of Zinc and Vitamin A Supplementation on Immune Responses in Indonesian Pre-Schoolers. Asia Pac. J. Clin. Nutr. 2020, 29, 732–742. [Google Scholar] [CrossRef]
- Long, K.Z.; Rosado, J.L.; Montoya, Y.; Solano, M.D.L.; Hertzmark, E.; DuPont, H.L.; Santos, J.I. Effect of Vitamin A and Zinc Supplementation on Gastrointestinal Parasitic Infections among Mexican Children. Pediatrics 2007, 120, e846–e855. [Google Scholar] [CrossRef]
- Smith, J.C.; Rao, D.; Makdani, D.; Hegar, A.; Douglass, L.W. Vitamin A and Zinc Supplementation of Preschool Children. J. Am. Coll. Nutr. 1999, 18, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Kolba, N.; Glahn, R.P.; Tako, E. Intra-Amniotic Administration (Gallus gallus) of Cicer arietinum and Lens culinaris Prebiotics Extracts and Duck Egg White Peptides Affects Calcium Status and Intestinal Functionality. Nutrients 2017, 9, 785. [Google Scholar] [CrossRef] [Green Version]
- Beasley, J.T.; Johnson, A.A.T.; Kolba, N.; Bonneau, J.P.; Glahn, R.P.; Ozeri, L.; Koren, O.; Tako, E. Nicotianamine-Chelated Iron Positively Affects Iron Status, Intestinal Morphology and Microbial Populations in Vivo (Gallus gallus). Sci. Rep. 2020, 10, 2297. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.; Tako, E. The In Ovo Feeding Administration (Gallus Gallus)—An Emerging In Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients 2018, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.P.; Martino, H.S.D.; Tako, E. Plant Origin Prebiotics Affect Duodenal Brush Border Membrane Functionality and Morphology, in Vivo (Gallus gallus). Food Funct. 2021, 12, 6157–6166. [Google Scholar] [CrossRef]
- Shojadoost, B.; Alizadeh, M.; Taha-Abdelaziz, K.; Shoja Doost, J.; Astill, J.; Sharif, S. In Ovo Inoculation of Vitamin A Modulates Chicken Embryo Immune Functions. J. Interferon Cytokine Res. 2021, 41, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef]
- Juste Contin Gomes, M.; Stampini Duarte Martino, H.; Tako, E. Effects of Iron and Zinc Biofortified Foods on Gut Microbiota In Vivo (Gallus gallus): A Systematic Review. Nutrients 2021, 13, 189. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.P.; Koren, O.; Tako, E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villagómez-Estrada, S.; Pérez, J.F.; Darwich, L.; Vidal, A.; Van Kuijk, S.; Melo-Durán, D.; Solà-Oriol, D. Effects of Copper and Zinc Sources and Inclusion Levels of Copper on Weanling Pig Performance and Intestinal Microbiota. J. Anim. Sci. 2020, 98, skaa117. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A Metabolism: An Update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Nutrient Requirements of Poultry: 9th Revised Edition, 1994, 9th ed.; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Cheng, J.; Kolba, N.; Sisser, P.; Turjeman, S.; Even, C.; Koren, O.; Tako, E. Intraamniotic Administration (Gallus gallus) of Genistein Alters Mineral Transport, Intestinal Morphology, and Gut Microbiota. Nutrients 2022, 14, 3473. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology in Vivo (Gallus gallus). Food Res. Int. 2020, 137, 109705. [Google Scholar] [CrossRef]
- Wang, X.; Kolba, N.; Liang, J.; Tako, E. Aleterations in Gut Microflora Populations and Brush Border Functionality Following Intra-Amniotic Administration (Gallus gallus) of Wheat Bran Prebiotic Extracts. Food Funct. 2019, 10, 4834–4843. [Google Scholar] [CrossRef]
- Agrizzi Verediano, T.; Stampini Duarte Martino, H.; Kolba, N.; Fu, Y.; Cristina Dias Paes, M.; Tako, E. Black Corn (Zea mays L.) Soluble Extract Showed Anti-Inflammatory Effects and Improved the Intestinal Barrier Integrity in Vivo (Gallus gallus). Food Res. Int. 2022, 157, 111227. [Google Scholar] [CrossRef]
- Dias, D.M.; Kolba, N.; Hart, J.J.; Ma, M.; Sha, S.T.; Lakshmanan, N.; Nutti, M.R.; Martino, H.S.D.; Glahn, R.P.; Tako, E. Soluble Extracts from Carioca Beans (Phaseolus vulgaris L.) Affect the Gut Microbiota and Iron Related Brush Border Membrane Protein Expression in Vivo (Gallus gallus). Food Res. Int. 2019, 123, 172–180. [Google Scholar] [CrossRef]
- Jackson, C.; Shukla, V.; Kolba, N.; Agarwal, N.; Padilla-Zakour, O.I.; Tako, E. Empire Apple (Malus domestica) Juice, Pomace, and Pulp Modulate Intestinal Functionality, Morphology, and Bacterial Populations In Vivo (Gallus gallus). Nutrients 2022, 14, 4955. [Google Scholar] [CrossRef]
- Tako, E.; Ferket, P.R.; Uni, Z. Changes in Chicken Intestinal Zinc Exporter MRNA Expression and Small Intestinal Functionality Following Intra-Amniotic Zinc-Methionine Administration. J. Nutr. Biochem. 2005, 16, 339–346. [Google Scholar] [CrossRef]
- Tako, E.; Bar, H.; Glahn, R.P. The Combined Application of the Caco-2 Cell Bioassay Coupled with In Vivo (Gallus gallus) Feeding Trial Represents an Effective Approach to Predicting Fe Bioavailability in Humans. Nutrients 2016, 8, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.M.; Liao, X.D.; Lin, L.U.; Zhang, L.Y.; Lin, X.I.; Luo, X.G. Effect of in Ovo Zinc Injection on the Embryonic Development, Tissue Zinc Contents, Antioxidation, and Related Gene Expressions of Broiler Breeder Eggs. J. Integr. Agric. 2018, 17, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Dufner-Beattie, J.; Wang, F.; Kuo, Y.M.; Gitschier, J.; Eide, D.; Andrews, G.K. The Acrodermatitis Enteropathica Gene ZIP4 Encodes a Tissue-Specific, Zinc-Regulated Zinc Transporter in Mice. J. Biol. Chem. 2003, 278, 33474–33481. [Google Scholar] [CrossRef] [Green Version]
- Hara, T.; Takeda, T.-A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological Roles of Zinc Transporters: Molecular and Genetic Importance in Zinc Homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Cousins, R.J. Gastrointestinal Factors Influencing Zinc Absorption and Homeostasis. Int. J. Vitam. Nutr. Res. 2010, 80, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Langmade, S.J.; Ravindra, R.; Daniels, P.J.; Andrews, G.K. The Transcription Factor MTF-1 Mediates Metal Regulation of the Mouse ZnT1 Gene. J. Biol. Chem. 2000, 275, 34803–34809. [Google Scholar] [CrossRef] [Green Version]
- Knez, M.; Tako, E.; Glahn, R.P.; Kolba, N.; De Courcy-Ireland, E.; Stangoulis, J.C.R. Linoleic Acid:Dihomo-γ-Linolenic Acid Ratio Predicts the Efficacy of Zn-Biofortified Wheat in Chicken (Gallus gallus). J. Agric. Food Chem. 2018, 66, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Blaner, W.S. Retinol and Retinyl Esters: Biochemistry and Physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honarbakhsh, M.; Ericsson, A.; Zhong, G.; Isoherranen, N.; Zhu, C.; Bromberg, Y.; van Buiten, C.; Malta, K.; Joseph, L.; Sampath, H.; et al. Impact of Vitamin A Transport and Storage on Intestinal Retinoid Homeostasis and Functions. J. Lipid Res. 2021, 62, 100046. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Wang, D.; Wen, J.; Chen, J. Vitamin A Deficiency Indicating as Low Expression of LRAT May Be a Novel Biomarker of Primary Hypertension. Clin. Exp. Hypertens. 2020, 43, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Zolfaghari, R. Regulation of Hepatic Retinol Metabolism: Perspectives from Studies on Vitamin A Status. J. Nutr. 2004, 134, S269–S275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amengual, J.; Golczak, M.; Palczewski, K.; von Lintig, J. Lecithin:Retinol Acyltransferase Is Critical for Cellular Uptake of Vitamin A from Serum Retinol-Binding Protein. J. Biol. Chem. 2012, 287, 24216–24227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Jeong, W.-I. Retinoic Acids and Hepatic Stellate Cells in Liver Disease. J. Gastroenterol. Hepatol. 2012, 27, 75–79. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A Membrane Receptor for Retinol Binding Protein Mediates Cellular Uptake of Vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Retinoid Homeostasis and Beyond: How Retinol Binding Protein 4 Contributes to Health and Disease. Nutrients 2022, 14, 1236. [Google Scholar] [CrossRef]
- De Medeiros, P.H.Q.S.; Pinto, D.V.; De Almeida, J.Z.; Rêgo, J.M.C.; Rodrigues, F.A.P.; Lima, A.Â.M.; Bolick, D.T.; Guerrant, R.L.; Oriá, R.B. Modulation of Intestinal Immune and Barrier Functions by Vitamin A: Implications for Current Understanding of Malnutrition and Enteric Infections in Children. Nutrients 2018, 10, 1128. [Google Scholar] [CrossRef] [Green Version]
- Neurath, M.F. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef] [Green Version]
- Rowart, P.; Wu, J.; Caplan, M.J.; Jouret, F. Implications of AMPK in the Formation of Epithelial Tight Junctions. Int. J. Mol. Sci. 2018, 19, 2040. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Tian, H.; Lin, J.; Xu, C.; Yuan, Y.; Gao, S.; Song, C.; Lv, P.; Mei, X. Zinc Promotes Autophagy and Inhibits Apoptosis through AMPK/MTOR Signaling Pathway after Spinal Cord Injury. Neurosci. Lett. 2020, 736, 135263. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, J.H.; Park, S.W.; Kim, H.J.; Chang, K.C. Retinoic Acid Inhibits Tissue Factor and HMGB1 via Modulation of AMPK Activity in TNF-α Activated Endothelial Cells and LPS-Injected Mice. Atherosclerosis 2015, 241, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.J. AMPK Improves Gut Epithelial Differentiation and Barrier Function via Regulating Cdx2 Expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the Gut Microbiome and Gastrointestinal Health in Humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Aschner, M.; Lei, X.G.; Gritsenko, V.A.; Santamaria, A.; Alekseenko, S.I.; Prakash, N.T.; Chang, J.-S.; Sizova, E.A.; Chao, J.C.J.; et al. Gut Microbiota as a Mediator of Essential and Toxic Effects of Zinc in the Intestines and Other Tissues. Int. J. Mol. Sci. 2021, 22, 13074. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Wang, Y.; Wang, L.; Yin, Y.; Yin, L.; Yang, H.; Yin, Y. Dietary Vitamin A Affects Growth Performance, Intestinal Development, and Functions in Weaned Piglets by Affecting Intestinal Stem Cells. J. Anim. Sci. 2020, 98, skaa020. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A.; Cocolin, L.; et al. Modulation of Intestinal Microbiota, Morphology and Mucin Composition by Dietary Insect Meal Inclusion in Free-Range Chickens. BMC Vet. Res. 2018, 14, 383. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.X.; Peng, K.M. Developmental Morphology of the Small Intestine of African Ostrich Chicks. Poult. Sci. 2008, 87, 2629–2635. [Google Scholar] [CrossRef]
- Laudadio, V.; Passantino, L.; Perillo, A.; Lopresti, G.; Passantino, A.; Khan, R.U.; Tufarelli, V. Productive Performance and Histological Features of Intestinal Mucosa of Broiler Chickens Fed Different Dietary Protein Levels. Poult. Sci. 2012, 91, 265–270. [Google Scholar] [CrossRef]
- Noah, T.K.; Donahue, B.; Shroyer, N.F. Intestinal Development and Differentiation. Exp. Cell Res. 2011, 317, 2702–2710. [Google Scholar] [CrossRef] [Green Version]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of Villus Height and Crypt Depth, and Enhancement of Disaccharide Digestion and Monosaccharide Absorption, in Piglets Fed on Cows’ Whole Milk after Weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [Green Version]
- Maares, M.; Keil, C.; Straubing, S.; Robbe-Masselot, C.; Haase, H. Zinc Deficiency Disturbs Mucin Expression, O-Glycosylation and Secretion by Intestinal Goblet Cells. Int. J. Mol. Sci. 2020, 21, 6149. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.-R.; Chang, S.-Y.; Chang, J.-H.; Kim, J.-O.; Yang, J.-Y.; Kim, C.-H.; Kweon, M.-N. Downregulation of Th17 Cells in the Small Intestine by Disruption of Gut Flora in the Absence of Retinoic Acid. J. Immunol. 2010, 184, 6799–6806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut Microbiome–Micronutrient Interaction: The Key to Controlling the Bioavailability of Minerals and Vitamins? BioFactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Zinc through the Three Domains of Life. J. Proteome Res. 2006, 5, 3173–3178. [Google Scholar] [CrossRef]
- Diao, H.; Yan, J.; Li, S.; Kuang, S.; Wei, X.; Zhou, M.; Zhang, J.; Huang, C.; He, P.; Tang, W. Effects of Dietary Zinc Sources on Growth Performance and Gut Health of Weaned Piglets. Front. Microbiol. 2021, 12, 3316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, L.; Zhao, M.L.; Wu, J.Q.; Wang, M.Y.; Cheng, X.C. Effects of Zinc-Methionine on Growth Performance, Intestinal Flora and Immune Function in Pigeon Squabs. Br. Poult. Sci. 2014, 55, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.W.; Wong, C.P.; Arnold, H.K.; Kasschau, K.; Gaulke, C.A.; Sharpton, T.J.; Ho, E. Age and Micronutrient Effects on the Microbiome in a Mouse Model of Zinc Depletion and Supplementation. PLoS ONE 2022, 17, e0275352. [Google Scholar] [CrossRef]
- Huda, M.N.; Ahmad, S.M.; Kalanetra, K.M.; Taft, D.H.; Alam, M.J.; Khanam, A.; Raqib, R.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Neonatal Vitamin A Supplementation and Vitamin A Status Are Associated with Gut Microbiome Composition in Bangladeshi Infants in Early Infancy and at 2 Years of Age. J. Nutr. 2019, 149, 1075–1088. [Google Scholar] [CrossRef] [Green Version]
- Bonakdar, M.; Czuba, L.C.; Han, G.; Zhong, G.; Luong, H.; Isoherrannen, N.; Vaishnava, S. Gut Commensals Expand Vitamin A Metabolic Capacity of the Mammalian Host. Cell Host Microbe 2022, 30, 1084–1092. [Google Scholar] [CrossRef]
- Woo, V.; Eshleman, E.M.; Hashimoto-Hill, S.; Whitt, J.; Wu, S.-E.; Engleman, L.; Rice, T.; Karns, R.; Qualls, J.E.; Haslam, D.B.; et al. Commensal Segmented Filamentous Bacteria-Derived Retinoic Acid Primes Host Defense to Intestinal Infection. Cell Host Microbe 2021, 29, 1744–1756.e5. [Google Scholar] [CrossRef]
- Lin, D.; Medeiros, D.M. The Microbiome as a Major Function of the Gastrointestinal Tract and Its Implication in Micronutrient Metabolism and Chronic Diseases. Nutr. Res. 2023, 112, 30–45. [Google Scholar] [CrossRef] [PubMed]



| Analyte | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Base Pair | GI Identifier |
|---|---|---|---|---|
| Zinc-Related | ||||
| ZnT1 | GGTAACAGAGCTGCCTTAACT | GGTAACAGAGCTGCCTTAACT | 105 | 54109718 |
| ZIP4 | TCTCCTTAGCAGACAATTGAG | GTGACAAACAAGTAGGCGAAAC | 95 | 107050877 |
| Δ6 desaturase | GGCGAAAGTCAGCCTATTGA | AGGTGGGAAGATGAGGAAGA | 93 | 261865208 |
| Vitamin A Metabolism | ||||
| CRBP2 | GGCTACATGGTTGCACTAGACA | AACCACCCGGTTATCGAGTC | 195 | NM_001277417.1 |
| LRAT | GATTTTGCCTATGGCGGCAG | TTGTCGGTCTGGAAGCTGAC | 197 | XM_420371.7 |
| RBP4 | TGCCACCAACACAGAACTCTC | CTTTGAAGCTGCTCACACGG | 149 | NM_205238.2 |
| STRA6 | GTGCGCTGAACTTTGTCTGC | TTCTTCCTGCTCCCGACCT | 116 | NM_001293202.2 |
| Inflammatory Response | ||||
| NF-κB | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG | 100 | 2130627 |
| TNF-α | GACAGCCTATGCCAACAAGTA | TTACAGGAAGGGCAACTCATC | 109 | 53854909 |
| IL-1β | CTCACAGTCCTTCGACATCTTC | TGTTGAGCCTCACTTTCTGG | 119 | 88702685 |
| Brush Border Membrane Functionality | ||||
| AMPK | CTCCACTTCCAGAAGGTTACTT | GCAGTAGCTATCGTTCATCCTATC | 140 | 427185 |
| OCLN | GTCTGTGGGTTCCTCATCGT | GTTCTTCACCCACTCCTCCA | 124 | 396026 |
| CDX2 | CCAGCAATGCCAGCATATTG | CGGTTTCTCCTTACCACTTCTT | 95 | 2246388 |
| 18S rRNA | GCAAGACGAACTAAAGCGAAAG | TCGGAACTACGACGGTATCT | 100 | 7262899 |
| Treatment Group | No Injection | H2O Only | Oil 0.5% | ZN | ZL | RN | RL | ZNRN | ZLRL |
|---|---|---|---|---|---|---|---|---|---|
| Plasma zinc (µg/mL) | 0.700 ± 0.322 a | 0.471 ± 0.006 a | 0.518 ± 0.113 a | 0.609 ± 0.067 a | 0.756 ± 0.101 a | 0.343 ± 0.037 a | 0.507 ± 0.118 a | 0.578 ± 0.058 a | 0.619 ± 0.116 a |
| Liver retinol (pmol/mg) | 16.099 ± 0.527 a | 16.605 ± 2.518 a | 19.020 ± 0.389 a | 18.234 ± 1.529 a | 17.023 ± 1.328 a | 26.590 ± 5.153 a | 21.467 ± 3.665 a | 21.577 ± 1.561 a | 19.226 ± 1.094 a |
| Treatment Group | Villi Goblet Diameter (µm) | Crypt Goblet Diameter (µm) | Crypt Goblet Cell Number | Crypt Goblet Cell Type | ||
|---|---|---|---|---|---|---|
| Acidic | Neutral | Mixed | ||||
| No injection | 3.37 ± 0.06 bc | 2.92 ± 0.06 d | 6.33 ± 0.25 ab | 5.52 ± 0.23 abc | 0.01 ± 0.01 d | 0.84 ± 0.08 a |
| H2O only | 3.15 ± 0.07 cd | 3.04 ± 0.07 cd | 7.11 ± 0.25 a | 6.59 ± 0.25 a | 0.01 ± 0.01 d | 0.51 ± 0.06 abc |
| Oil 0.5% | 2.83 ± 0.07 d | 2.90 ± 0.07 d | 4.53 ± 0.19 c | 3.84 ± 0.19 e | 0.07 ± 0.02 bcd | 0.62 ± 0.06 ab |
| ZN | 2.93 ± 0.07 d | 2.79 ± 0.07 d | 3.23 ± 0.14 d | 2.60 ± 0.14 f | 0.15 ± 0.03 bc | 0.55 ± 0.07 bc |
| ZL | 3.03 ± 0.07 d | 2.80 ± 0.06 d | 4.64 ± 0.22 c | 4.40 ± 0.35 de | 0.07 ± 0.03 cd | 0.27 ± 0.04 c |
| RN | 2.90 ± 0.07 d | 3.09 ± 0.07 cd | 5.91 ± 0.26 ab | 5.40 ± 0.25 abcd | 0.08 ± 0.02 bcd | 0.55 ± 0.08 bc |
| RL | 3.70 ± 0.08 b | 3.58 ± 0.07 ab | 5.51 ± 0.23 bc | 5.01 ± 0.23 bcd | 0.17 ± 0.03 ab | 0.33 ± 0.05 c |
| ZNRN | 4.12 ± 0.08 a | 3.65 ± 0.05 a | 5.28 ± 0.23 bc | 4.82 ± 0.23 bcde | 0.09 ± 0.03 bcd | 0.41 ± 0.06 c |
| ZLRL | 4.08 ± 0.07 a | 3.29 ± 0.06 bc | 3.15 ± 0.16 d | 2.39 ± 0.16 f | 0.32 ± 0.05 a | 0.51 ± 0.06 bc |
| Treatment Group | No Injection | H2O Only | Oil 0.5% | ZN | ZL | RN | RL | ZNRN | ZLRL |
|---|---|---|---|---|---|---|---|---|---|
| Crypt Paneth Cell Number | 1.17 ± 0.06 de | 1.31 ± 0.04 bcd | 1.36 ± 0.05 abcd | 1.49 ± 0.05 a | 1.32 ± 0.04 abc | 1.07 ± 0.02 e | 1.38 ± 0.04 ab | 1.23 ± 0.03 bcde | 1.18 ± 0.03 de |
| Paneth Cell Diameter (µm) | 1.69 ± 0.03 a | 1.56 ± 0.03 abc | 1.46 ± 0.03 cde | 1.64 ± 0.03 a | 1.33 ± 0.02 e | 1.59 ± 0.03 ab | 1.41 ± 0.03 de | 1.47 ± 0.02 bcd | 1.59 ± 0.03 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, C.; Kolba, N.; Tako, E. Assessing the Interactions between Zinc and Vitamin A on Intestinal Functionality, Morphology, and the Microbiome In Vivo (Gallus gallus). Nutrients 2023, 15, 2754. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15122754
Jackson C, Kolba N, Tako E. Assessing the Interactions between Zinc and Vitamin A on Intestinal Functionality, Morphology, and the Microbiome In Vivo (Gallus gallus). Nutrients. 2023; 15(12):2754. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15122754
Chicago/Turabian StyleJackson, Cydney, Nikolai Kolba, and Elad Tako. 2023. "Assessing the Interactions between Zinc and Vitamin A on Intestinal Functionality, Morphology, and the Microbiome In Vivo (Gallus gallus)" Nutrients 15, no. 12: 2754. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15122754