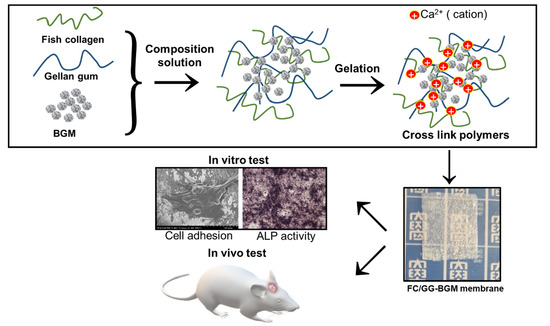
Biomedical Membrane of Fish Collagen/Gellan Gum Containing Bone Graft Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FC/GG and FC/GG-BGM Membranes
2.3. Characterization of the FC/GG-BGM Membrane
2.4. Measurement of Mechanical Properties
2.5. Alkaline Phosphatase (ALP) Activity
2.6. In Vitro Cytotoxicity Assay
2.7. Experimental Animal Model and Surgical Procedure
2.8. Micro-Computed Tomography (Micro-Ct) Analysis
2.9. Histological Preparation and Evaluation
2.10. Statistical Analysis
3. Results
3.1. Morphology and Mechanical Properties of FC/GG-BGM Membrane
3.2. FTIR Analysis of the FC/GG-BGM Membrane
3.3. XRD of the FC/GG-BGM Membrane
3.4. AFM and Mechanical Analysis of the FC/GG-BGM Membrane
3.5. Cytotoxicity of the FC/GG-BGM Membrane
3.6. ALP Activity of the FC/GG-BGM Membrane
3.7. Micro-CT Imaging
3.8. Histological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Wang, J.; Yang, Y.; Yin, Q. Bone defects are repaired by enhanced osteogenic activity of the induced membrane: A case report and literature review. BMC Musculoskelet. Disord. 2021, 22, 447. [Google Scholar] [CrossRef] [PubMed]
- Aghali, A. Craniofacial bone tissue engineering: Current approaches and potential therapy. Cells 2021, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M.; Gutow, A.P.; Sharpe, F. The management of bone defects of the forearm after trauma. Hand Clin. 1999, 15, 299–318. [Google Scholar] [CrossRef]
- Van de Vijfeijken, S.E.C.M.; Groot, C.; Ubbink, D.T.; Vandertop, W.P.; Depauw, P.R.A.M.; Nout, E.; Becking, A.G.; CranioSafe Group. Factors related to failure of autologous cranial reconstructions after decompressive craniectomy. J. Craniomaxillofac. Surg. 2019, 47, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Dandriyal, R.; Gupta, A.; Pant, S.; Baweja, H.H. Surgical management of ameloblastoma: Conservative or radical approach. Natl. J. Maxillofac. Surg. 2011, 2, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic material for bone, periodontal, and dental tissue regeneration: Where are we now, and where are we heading next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Schwarz, F.; Jung, R.E.; Fienitz, T.; Wieland, M.; Becker, J.; Sager, M. Impact of guided bone regeneration and defect dimension on wound healing at chemically modified hydrophilic titanium implant surfaces: An experimental study in dogs. J. Clin. Periodontol. 2010, 37, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Mellgren, T.; Trbakovic, A.; Thor, A.; Ekman, S.; Ley, C.; Öhman-Mägi, C.; Johansson, P.H.; Jensen-Waern, M.; Hedenqvist, P. Guided bone tissue regeneration using a hollow calcium phosphate-based implant in a critical size rabbit radius defect. Biomed. Mater. 2021, 16, 035018. [Google Scholar]
- Liang, H.; Yin, J.; Man, K.; Yang, X.B.; Calciolari, E.; Donos, N.; Russell, S.J.; Wood, D.J.; Tronci, G. A long-lasting guided bone regeneration membrane from sequentially functionalised photoactive atelocollagen. Acta Biomater. 2022, 140, 190–205. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Zaky, S.H.; Schoedel, K.; Li, H.; Sant, V.; Beniash, E.; Sfeir, C.; Stolz, D.B.; Sant, S. Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration. Acta Biomater. 2020, 112, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.G.; Yu, J.A.; Choi, S.H.; Lee, D.W. Clinical, radiographic, and histomorphometric evaluation of a vertical ridge augmentation procedure using a titanium-reinforced microporous expanded polytetrafluoroethylene Membrane: A prospective case series with 1-year follow-up. Materials 2021, 14, 3828. [Google Scholar] [CrossRef] [PubMed]
- Tunthasen, R.; Pripatnanont, P.; Meesane, J. Fabrication and characterization of a semi-rigid shell barrier system made of polycaprolactone and biphasic calcium phosphate: A novel barrier system for bone regeneration. J. Mech. Behav. Biomed. Mater. 2021, 124, 104841. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Mei, D.; Lin, X.; Zhang, L.; Gao, J.; Li, X.; Zhu, X.; Jin, Q.; Zhang, S.; Xu, H.; et al. A synthetic biodegradable polymer membrane for guided bone regeneration in bone defect. J. Biomed. Nanotechnol. 2021, 17, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhata, M.; Takayama, T.; Yamamoto, T.; Ozawa, Y.; Senoo, M.; Ozaki, M.; Yamano, S.; Sato, S. Real-time assessment of guided bone regeneration in critical size mandibular bone defects in rats using collagen membranes with adjunct fibroblast growth factor-2. J. Dent. Sci. 2021, 16, 1170–1181. [Google Scholar] [CrossRef]
- Elango, J.; Saravanakumar, K.; Rahman, S.U.; Henrotin, Y.; Regenstein, J.M.; Wu, W.; Bao, B. Chitosan-collagen 3D matrix mimics trabecular bone and regulates RANKL-mediated paracrine cues of differentiated osteoblast and mesenchymal stem cells for bone marrow macrophage-derived osteoclastogenesis. Biomolecules 2019, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- De Brito Bezerra, B.; Mendes Brazão, M.A.; de Campos, M.L.; Casati, M.Z.; Sallum, E.A.; Sallum, A.W. Association of hyaluronic acid with a collagen scaffold may improve bone healing in critical-size bone defects. Clin. Oral Implants Res. 2012, 23, 938–942. [Google Scholar] [CrossRef]
- Santosh Kumar, B.B.; Aruna, D.R.; Vinayak Gowda, S.; Sushama Galagali, R. Evaluation of a bioresorbable collagen membrane of fish origin in the treatment of periodontal intrabony defects: A prospective clinical study. Dent. Res. J. 2013, 10, 225–231. [Google Scholar] [CrossRef]
- Nagai, N.; Yunoki, S.; Suzuki, T.; Sakata, M.; Tajima, K.; Munekata, M. Application of cross-linked salmon atelocollagen to the scaffold of human periodontal ligament cells. J. Biosci. Bioeng. 2004, 97, 389–394. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.A. Current state of bone replacement grafting materials for dental implants. Compend. Contin. Educ. Dent. 2021, 42, 466–467. [Google Scholar] [PubMed]
- Misch, C.E.; Dietsh, F. Bone-grafting materials in implant dentistry. Implant. Dent. 1993, 2, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Lee, J.H.; Oh, S.H.; Ham, B.D.; Chung, S.M.; Lee, J.K.; Ku, J.K. Bone graft materials for current implant dentistry. J. Dental Implant Res. 2020, 39, 1–10. [Google Scholar] [CrossRef]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation. Materials 2015, 27, 2953–2993. [Google Scholar] [CrossRef]
- Han, J.Y.; Shin, S.I.; Herr, Y.; Kwon, Y.H.; Chung, J.H. The effects of bone grafting material and a collagen membrane in the ridge splitting technique: An experimental study in dogs. Clin. Oral Implants Res. 2011, 22, 1391–1398. [Google Scholar] [CrossRef]
- Fushimi, H.; Hiratsuka, T.; Okamura, A.; Ono, Y.; Ogura, I.; Nishimura, I. Recombinant collagen peptide as a versatile bone graft biomaterials. Comm. Mat. 2020, 1, 87. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Martins, L.; Picciochi, R.; Malafaya, P.B.; Sousa, R.A.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 93, 852–863. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Choi, J.H.; Kim, P.; Youn, J.; Song, J.E.; Motta, A.; Migliaresi, C.; Khang, G. Preparation and evaluation of gellan gum hydrogel reinforced with silk fibers with enhanced mechanical and biological properties for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2021, 15, 936–947. [Google Scholar] [CrossRef]
- Wang, H.L.; Carroll, M.J. Guided bone regeneration using bone grafts and collagen membranes. Quintessence Int. 2001, 32, 504–515. [Google Scholar]
- Takata, T.; Wang, H.L.; Miyauchi, M. Migration of osteoblastic cells on various guided bone regeneration membranes. Clin. Oral Implants Res. 2001, 12, 332–338. [Google Scholar] [PubMed]
- Safandowska, M.; Pietrucha, K. Effect of fish collagen modification on its thermal and rheological properties. Int. J. Biol. Macromol. 2013, 53, 32–37. [Google Scholar] [PubMed]
- Nagai, N.; Nakayama, Y.; Nishi, S.; Munekata, M. Development of novel covered stents using salmon collagen. J. Artif. Organs 2009, 12, 61–66. [Google Scholar] [PubMed]
- Sugiura, H.; Yunoki, S.; Kondo, E.; Ikoma, T.; Tanaka, J.; Yasuda, K. In vivo biological responses and bioresorption of tilapia scale collagen as a potential biomaterial. J. Biomater. Sci. Polym. Ed. 2009, 20, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, W.; Song, C.; Moon, B.K.; Yoon, S.J.; Neves, N.M.; Reis, R.L.; Khang, G. Application of gellan gum-based scaffold for regenerative medicine. Adv. Exp. Med. Biol. 2020, 1249, 15–37. [Google Scholar] [PubMed]
- Kim, J.; Jang, J.W.; Lee, C.M.; Lee, K.Y. Development of bone generation scaffolds composed of croaker swim bladder and gellan gum. Polymer 2017, 41, 324–330. [Google Scholar] [CrossRef]
- Magan, A.; Ripamonti, U. Geometry of porous hydroxyapatite implants influences osteogenesis in baboons (Papio lIrsinlls). J. Craniofacial Surg. 1996, 7, 71–78. [Google Scholar] [CrossRef]
- Ripamonti, A.; Ma, S.; Reddi, A.H. The critical role of geometry of porous hydroxyapatite delivery system in induction of bone by osteogenin, a bone morphogenetic protein. Matrix 1992, 12, 202–212. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, C.-M.; Moon, S.-Y.; Jeong, Y.-I.; Kim, C.S.; Lee, S.-Y. Biomedical Membrane of Fish Collagen/Gellan Gum Containing Bone Graft Materials. Materials 2022, 15, 2954. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15082954
Kim J, Lee C-M, Moon S-Y, Jeong Y-I, Kim CS, Lee S-Y. Biomedical Membrane of Fish Collagen/Gellan Gum Containing Bone Graft Materials. Materials. 2022; 15(8):2954. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15082954
Chicago/Turabian StyleKim, Jin, Chang-Moon Lee, Seong-Yong Moon, Young-IL Jeong, Chun Sung Kim, and Sook-Young Lee. 2022. "Biomedical Membrane of Fish Collagen/Gellan Gum Containing Bone Graft Materials" Materials 15, no. 8: 2954. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15082954