Nanoplastics Penetrate Human Bronchial Smooth Muscle and Small Airway Epithelial Cells and Affect Mitochondrial Metabolism
Abstract
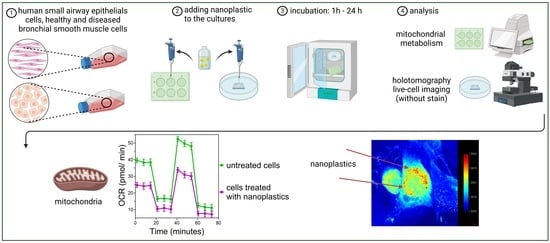
:1. Introduction
2. Results
2.1. Visualization of NPs
2.2. Mitochondrial Metabolism
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Cell Cultures
4.3. Nanoplastics
4.4. Holotomography Microscope
4.5. Mitochondrial Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernández-León, S.; Palma, A.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Galloway, T.S. Ingestion of Nanoplastics and Microplastics by Pacific Oyster Larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef] [PubMed]
- Filella, M. Questions of size and numbers in environmental research on microplastics: Methodological and conceptual aspects. Environ. Chem. 2015, 12, 527. [Google Scholar] [CrossRef]
- Heilig, S.; Adams, D.R.; Zaenglein, A.L. Persistent allergic contact dermatitis to plastic toilet seats. Pediatr. Dermatol. 2011, 28, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2021, 56, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Oddone, E.; Modonesi, C.; Gatta, G. Occupational exposures and colorectal cancers: A quantitative overview of epidemiological evidence. World J. Gastroenterol. 2014, 20, 12431–12444. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, H.A.; Juel, K.; Olsen, J.; Lynge, E. Exposure to styrene and chronic health effects: Mortality and incidence of solid cancers in the Danish reinforced plastics industry. Occup. Environ. Med. 1995, 52, 320–327. [Google Scholar] [CrossRef]
- González-Acedo, A.; García-Recio, E.; Illescas-Montes, R.; Ramos-Torrecillas, J.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J. Evidence from in vitro and in vivo studies on the potential health repercussions of micro- and nanoplastics. Chemosphere 2021, 280, 130826. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef]
- Auguet, T.; Bertran, L.; Barrientos-Riosalido, A.; Fabregat, B.; Villar, B.; Aguilar, C.; Sabench, F. Are Ingested or Inhaled Microplastics Involved in Nonalcoholic Fatty Liver Disease? Int. J. Environ. Res. Public Health 2022, 19, 13495. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Plastic pollution and potential solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 8 February 2024).
- Heddagaard, F.E.; Møller, P. Hazard assessment of small-size plastic particles: Is the conceptual framework of particle toxicology useful? Food Chem. Toxicol. 2019, 136, 111106. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Camoretti-Mercado, B.; Lockey, R.F. Airway smooth muscle pathophysiology in asthma. J. Allergy Clin. Immunol. 2021, 147, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Hellings, P.W.; Steelant, B. Epithelial barriers in allergy and asthma. J. Allergy Clin. Immunol. 2020, 145, 1499–1509. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, H.; Zhang, J.; Liu, Y.; Zhong, W.; Chen, W.; Lu, Y.; Qiao, Y.; Zhao, H.; Meng, X.; et al. HDM induce airway epithelial cell ferroptosis and promote inflammation by activating ferritinophagy in asthma. FASEB J. 2022, 36, e22359. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q. Using Seahorse Machine to Measure OCR and ECAR in Cancer Cells. In Cancer Metabolism; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1928, pp. 353–363. [Google Scholar] [CrossRef]
- Leung, D.T.H.; Chu, S. Measurement of Oxidative Stress: Mitochondrial Function Using the Seahorse System. In Preeclampsia; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1710, pp. 285–293. [Google Scholar] [CrossRef]
- Baczewska, M.; Eder, K.; Ketelhut, S.; Kemper, B.; Kujawińska, M. Refractive Index Changes of Cells and Cellular Compartments Upon Paraformaldehyde Fixation Acquired by Tomographic Phase Microscopy. Cytom. Part A 2020, 99, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bai, B.; Ryu, D.; Liu, T.; Lee, C.; Luo, Y.; Lee, M.J.; Huang, L.; Shin, J.; Zhang, Y.; et al. Artificial intelligence-enabled quantitative phase imaging methods for life sciences. Nat. Methods 2023, 20, 1645–1660. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Kim, J.H.; Min, H.S.; Jang, S.; Hong, J.; Choi, B.K.; Shin, J.; Chung, K.S.; Park, Y.R. Three-dimensional label-free morphology of CD8 + T cells as a sepsis biomarker. Light Sci. Appl. 2023, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Bresci, A.; Kim, J.H.; Ghislanzoni, S.; Manetti, F.; Wu, L.; Vernuccio, F.; Ceconello, C.; Sorrentino, S.; Barman, I.; Bongarzone, I.; et al. Noninvasive morpho-molecular imaging reveals early therapy-induced senescence in human cancer cells. Sci. Adv. 2023, 9, eadg6231. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Oh, S.; Kirschner, M.W. The uniformity and stability of cellular mass density in mammalian cell culture. Front. Cell Dev. Biol. 2022, 10, 1017499. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Barrios, A.C.; Henry, T.B.; Johnson, M.E.; Koelmans, A.A.; Montoro Bustos, A.R.; Matheson, J.; Roesslein, M.; Zhao, J.; Xing, B. Potential Artifacts and Control Experiments in Toxicity Tests of Nanoplastic and Microplastic Particles. Environ. Sci. Technol. 2022, 56, 15192–15206. [Google Scholar] [CrossRef] [PubMed]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. In Vitro 2019, 61, 104610. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. In Vitro 2016, 31, 126–136. [Google Scholar] [CrossRef]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef]
- Busch, M.; Bredeck, G.; Kämpfer, A.A.M.; Schins, R.P.F. Investigations of acute effects of polystyrene and polyvinyl chloride micro- and nanoplastics in an advanced in vitro triple culture model of the healthy and inflamed intestine. Environ. Res. 2021, 193, 110536. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiriccò, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Kim, C.E.; Shin, Y.H.; Kim, J.K.; Pack, C.G. Influence of Chemical and Genetic Manipulations on Cellular Organelles Quantified by Label-Free Optical Diffraction Tomography. Anal. Chem. 2023, 95, 13478–13487. [Google Scholar] [CrossRef] [PubMed]
- Ganderton, T.R.; Ghete, D.; Hogg, K.; Park, G.J.; Baumann, C.G.; Wilkinson, A.J.; Pryor, P.R. Commonality of Virulence-Promoting Function in Rhodococcus equi Virulence Associated Proteins (Vaps). Cell. Microbiol. 2023, 2023, 9141112. [Google Scholar] [CrossRef]
- Oh, S.; Lee, C.; Yang, W.; Li, A.; Mukherjee, A.; Basan, M.; Ran, C.; Yin, W.; Tabin, C.J.; Fu, D.; et al. Protein and lipid mass concentration measurement in tissues by stimulated Raman scattering microscopy. Proc. Natl. Acad. Sci. USA 2022, 119, e2117938119. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Hirayama, K.; Matsukawa, Y.; Takemura, M.; Umemura, K. Digital Holographic Microscopy of Living Hela Cells before and after Enzyme Treatments. In Proceedings of the 6th International Conference on Biomedical Engineering and Applications, New York, NY, USA, 13–15 May 2022; pp. 127–129. [Google Scholar] [CrossRef]
- Kang, S.H.; Shin, Y.S.; Lee, D.H.; Park, I.S.; Kim, S.K.; Ryu, D.; Park, Y.; Byun, S.H.; Choi, J.H.; Hong, S.J. Interactions of Nanoparticles with Macrophages and Feasibility of Drug Delivery for Asthma. Int. J. Mol. Sci. 2022, 23, 1622. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Marz, A.; Wiemann, M.; Rauen, U.; Kemper, B.; Schnekenburger, J. Morphological alterations in primary hepatocytes upon nanomaterial incubation assessed by digital holographic microscopy and holotomography. In Proceedings of the Quantitative Phase Imaging VIII, San Francisco, CA, USA, 22 January–28 February 2022; Volume 119700H. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.A.; McCarty, P.J.; Lane, A.L.; Singh, I.; Karim, M.A.; Rose, S.; Frye, R.E. Time dependent changes in the bioenergetics of peripheral blood mononuclear cells: Processing time, collection tubes and cryopreservation effects. Am. J. Transl. Res. 2022, 14, 1628–1639. [Google Scholar]
- Gibellini, L.; De Biasi, S.; Paolini, A.; Borella, R.; Boraldi, F.; Mattioli, M.; Lo Tartaro, D.; Fidanza, L.; Caro-Maldonado, A.; Meschiari, M.; et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol. Med. 2020, 12, e13001. [Google Scholar] [CrossRef]
- Barshtein, G.; Livshits, L.; Shvartsman, L.D.; Shlomai, N.O.; Yedgar, S.; Arbell, D. Polystyrene Nanoparticles Activate Erythrocyte Aggregation and Adhesion to Endothelial Cells. Cell Biochem. Biophys. 2015, 74, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Halimu, G.; Zhang, Q.; Liu, L.; Zhang, Z.; Wang, X.; Gu, W.; Zhang, B.; Dai, Y.; Zhang, H.; Zhang, C.; et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J. Hazard. Mater. 2022, 430, 128485. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Yi, Y.; Moon, S.; Yoon, H.; Park, Y.S. Impact of Micro- and Nanoplastics on Mitochondria. Metabolites 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ. Pollut. 2022, 300, 118986. [Google Scholar] [CrossRef] [PubMed]
- Gettings, S.M.; Timbury, W.; Dmochowska, A.; Sharma, R.; MacKenzie, L.E.; Miquelard-Garnier, G.; Bourbia, N. Polyethylene terephthalate (PET) micro- and nanoplastic particles affect the mitochondrial efficiency of human brain vascular pericytes without inducing oxidative stress. bioRxiv 2023. [Google Scholar] [CrossRef]
- Brandts, I.; Solà, R.; Garcia-Ordoñez, M.; Gella, A.; Quintana, A.; Martin, B.; Esteve-Codina, A.; Telesab, M.; Roher, N. Polystyrene nanoplastics target lysosomes interfering with lipid metabolism through the PPAR system and affecting macrophage functionalization. Environ. Sci. Nano 2023, 10, 2245–2258. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska, E.; Chaszczewska-Markowska, M.; Ghete, D.; Jutel, M.; Zemelka-Wiacek, M. Nanoplastics Penetrate Human Bronchial Smooth Muscle and Small Airway Epithelial Cells and Affect Mitochondrial Metabolism. Int. J. Mol. Sci. 2024, 25, 4724. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25094724
Winiarska E, Chaszczewska-Markowska M, Ghete D, Jutel M, Zemelka-Wiacek M. Nanoplastics Penetrate Human Bronchial Smooth Muscle and Small Airway Epithelial Cells and Affect Mitochondrial Metabolism. International Journal of Molecular Sciences. 2024; 25(9):4724. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25094724
Chicago/Turabian StyleWiniarska, Ewa, Monika Chaszczewska-Markowska, Daniel Ghete, Marek Jutel, and Magdalena Zemelka-Wiacek. 2024. "Nanoplastics Penetrate Human Bronchial Smooth Muscle and Small Airway Epithelial Cells and Affect Mitochondrial Metabolism" International Journal of Molecular Sciences 25, no. 9: 4724. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms25094724