Formulation and Characterization of Chitosan-Based Mixed-Matrix Scaffold for Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
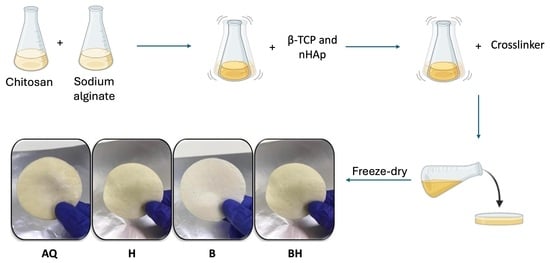
2.2. Scaffold Preparation
2.3. Characterization Methods
3. Results
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. X-ray Diffraction (XRD)
3.3. Scanning Electron Microscopy (SEM)
3.4. Porosity Determination by Ethanol Method
3.5. Positron Annihilation Lifetime Spectroscopy
3.6. Thermogravimetric Analysis
3.7. Mechanical Tests
3.8. Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lidgren, L.; Gomez-Barrena, E.; Duda, G.N.; Puhl, W.; Carr, A. European musculoskeletal health and mobility in Horizon 2020. Bone Jt. Res. 2014, 3, 48–50. [Google Scholar] [CrossRef]
- Tomé-Bermejo, F.; Piñera, A.R.; Alvarez, L. Osteoporosis and the management of spinal degenerative disease (II). Arch. Bone Jt. Surg. 2017, 5, 363–374. [Google Scholar]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of Silicon Compounds on Biomineralization, Osteogenesis, and Hard Tissue Formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef]
- Chaudhary, C.; Garg, T. Scaffolds: A novel carrier and potential wound healer. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 277–321. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-vázquez, M.; Vega-ruiz, B.; Ramos-zúñiga, R.; Saldaña-koppel, D.A.; Quiñones-olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J.; Chrzanowska, E. pH-responsive chitosan/alginate polyelectrolyte complex membranes reinforced by tripolyphosphate. Eur. Polym. J. 2018, 101, 282–290. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107 Pt A, 678–688. [Google Scholar] [CrossRef]
- Shangari, N.; O’Brien, P.J. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem. Pharmacol. 2004, 68, 1433–1442. [Google Scholar] [CrossRef]
- Wang, L.; Stegemann, J.P. Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomater. 2011, 7, 2410–2417. [Google Scholar] [CrossRef]
- Bròdano, G.B.; Giavaresi, G.; Lolli, F.; Salamanna, F.; Parrilli, A.; Martini, L.; Griffoni, C.; Greggi, T.; Arcangeli, E.; Pressato, D.; et al. Hydroxyapatite-based biomaterials versus autologous bone graft in spinal fusion: An in vivo animal study. Spine 2014, 39, E661–E668. [Google Scholar] [CrossRef]
- Wongwitwichot, P.; Kaewsrichan, J.; Chua, K.; Ruszymah, B.H. Comparison of TCP and TCP/HA Hybrid Scaffolds for Osteoconductive Activity. Open Biomed. Eng. J. 2014, 4, 279–285. [Google Scholar] [CrossRef]
- Schimandle, J.H.; Boden, S.D. Bone substitutes for lumbar fusion: Present and future. Oper. Technol. Orthop. 1997, 7, 60–67. [Google Scholar] [CrossRef]
- Baghbani, F.; Moztarzadeh, F.; Nazari, A.G.; Kamran, A.H.R.; Tondnevis, F.; Nezafati, N.; Gholipourmalekabadi, M.; Mozafari, M. Biological Response of Biphasic Hydroxyapatite/Tricalcium Phosphate Scaffolds Intended for Low Load-Bearing Orthopaedic Applications. Adv. Compos. Lett. 2012, 21, 096369351202100. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Y.; Cui, Y.; Jing, X.; Zhang, P.; Chen, X. The nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with l-lactic acid oligomer for bone repair. Acta Biomater. 2009, 5, 2680–2692. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, T.; Cui, L.; Wang, Y.; Zhang, P.; Chen, X. Improved cellular infiltration into 3D interconnected microchannel scaffolds formed by using melt-spun sacrificial microfibers. RSC Adv. 2016, 6, 2131–2134. [Google Scholar] [CrossRef]
- Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1996, 374, 235–244. [Google Scholar] [CrossRef]
- Jean, Y.C.; Van Horn, J.D.; Hung, W.-S.; Lee, K.-R. Perspective of Positron Annihilation Spectroscopy in Polymers. Macromolecules 2013, 46, 7133–7145. [Google Scholar] [CrossRef]
- Gong, W.; Mai, Y.; Zhou, Y.; Qi, N.; Wang, B.; Yan, D. Effect of the Degree of Branching on Atomic-Scale Free Volume in Hyperbranched Poly[3-ethyl-3-(hydroxymethyl)oxetane]. A Positron Study. Macromolecules 2005, 38, 9644–9649. [Google Scholar] [CrossRef]
- Monge, M.A.; Díaz, J.A.; Pareja, R. Strain-Induced Changes of Free Volume Measured by Positron Lifetime Spectroscopy in Ultrahigh Molecular Weight Polyethylene. Macromolecules 2004, 37, 7223–7230. [Google Scholar] [CrossRef]
- Ferreira Marques, M.F.; Gordo, P.M.; Kajcsos, Z.; Lopes Gil, C.; de Lima, A.P.; Queiroz, D.P.; de Pinho, M.N. Positron studies of the temperature-dependence of free volumes in Polydimethylsiloxane/poly(propylene oxide) urethane/urea membranes. Radiat. Phys. Chem. 2007, 76, 129–133. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate-chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Yang, Q.; Dou, F.; Liang, B.; Shen, Q. Studies of cross-linking reaction on chitosan fiber with glyoxal. Carbohydr. Polym. 2005, 59, 205–210. [Google Scholar] [CrossRef]
- Liao, J.; Li, Y.; Li, H.; Liu, J.; Xie, Y.; Wang, J.; Zhang, Y. Preparation, bioactivity and mechanism of nano-hydroxyapatite/sodium alginate/chitosan bone repair material. J. Appl. Biomater. Funct. Mater. 2018, 16, 28–35. [Google Scholar] [CrossRef]
- Bi, Y.; Guang, L.; Ting, Z.; Deng, S.T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 576–583. [Google Scholar] [CrossRef]
- Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.C.; Silvestros, S.; Sagnou, M. Synthesis of hydroxyapatite, β-Tricalcium phosphate and biphasic calcium phosphate particles to act as local delivery carriers of curcumin: Loading, release and in vitro studies. Materials 2018, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Kolassa, N.; Punzengruber, C.; Suko, J.; Makinose, M. Mechanism of calcium-independent phosphorylation of sarcoplasmic reticulum ATPase by orthophosphate. Evidence of magnesium-phosphoprotein formation. FEBS Lett. 1979, 108, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Thacker, A.; Sperger, D.M.; Boni, R.L.; Buckner, I.S.; Velankar, S.; Munson, E.J.; Block, L.H. Relevance of rheological properties of sodium alginate in solution to calcium alginate gel properties. AAPS PharmSciTech 2011, 12, 453–460. [Google Scholar] [CrossRef]
- Hussain, R.; Iman, M.; Maji, T.K. Determination of Degree of Deacetylation of Chitosan and Their effect on the Release Behavior of Essential Oil from Chitosan and Chitosan—Gelatin Complex Microcapsules. Int. J. Adv. Eng. Appl. 2014, 37, 69–77. [Google Scholar]
- Chaudhary, D.; Went, M.R.; Nakagawa, K.; Buckman, S.J.; Sullivan, J.P. Molecular pore size characterization within chitosan biopolymer using positron annihilation lifetime spectroscopy. Mater. Lett. 2010, 64, 2635–2637. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Sundarrajan, P.; Eswaran, P.; Marimuthu, A.; Subhadra, L.B.; Kannaiyan, P. One pot synthesis and characterization of alginate stabilized semiconductor nanoparticles. Bull. Korean Chem. Soc. 2012, 33, 3218–3224. [Google Scholar] [CrossRef]
- Nair, R.M.; Bindhu, B.; Reena, V.L. A polymer blend from Gum Arabic and Sodium Alginate—Preparation and characterization. J. Polym. Res. 2020, 27, 154. [Google Scholar] [CrossRef]
- Danilchenko, S.N.; Kukharenko, O.G.; Moseke, C.; Protsenko, I.Y.; Sukhodub, L.F.; Sulkio-Cleff, B. Determination of the bone mineral crystallite size and lattice strain from diffraction line broadening. Cryst. Res. Technol. 2002, 37, 1234–1240. [Google Scholar] [CrossRef]
- Danilchenko, S.N.; Kalinkevich, O.V.; Pogorelov, M.V.; Kalinkevich, A.N.; Sklyar, A.M.; Kalinichenko, T.G.; Ilyashenko, V.Y.; Starikov, V.V.; Bumeyster, V.I.; Sikora, V.Z.; et al. Characterization and in vivo evaluation of chitosan-hydroxyapatite bone scaffolds made by one step coprecipitation method. J. Biomed. Mater. Res. Part A 2011, 96, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.H.; Lee, E.J.; Yoon, B.H.; Shin, D.S.; Kim, H.E.; Oh, J.S. Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J. Biomed. Mater. Res. Part A 2009, 88, 569–580. [Google Scholar] [CrossRef]
- Kucharska, M.; Butruk, B.; Walenko, K.; Brynk, T.; Ciach, T. Fabrication of in-situ foamed chitosan/β-TCP scaffolds for bone tissue engineering application. Mater. Lett. 2012, 85, 124–127. [Google Scholar] [CrossRef]
- Rajkumar, M.; Meenakshisundaram, N.; Rajendran, V. Development of nanocomposites based on hydroxyapatite/sodium alginate: Synthesis and characterisation. Mater. Charact. 2011, 62, 469–479. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ito, K.; Oka, T.; Hirata, K. Positronium chemistry in porous materials. Radiat. Phys. Chem. 2007, 76, 224–230. [Google Scholar] [CrossRef]
- Eldrup, M.; Lightbody, D.; Sherwood, J.N. The temperature dependence of positron lifetimes in solid pivalic acid. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Ingram, A.; Shpotyuk, O. Free Volume Structure of Acrylic-Type Dental Nanocomposites Tested with Annihilating Positrons. Nanoscale Res. Lett. 2016, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Bugnicourt, L.; Merida, D.; Goiriena-Goikoetxea, M.; Unzueta, I.; Sanchez-Eugenia, R.; Garcia, J.A.; Plazaola, F.; Contera, S. Sub-nanoscale free volume and local elastic modulus of chitosan–carbon nanotube biomimetic nanocomposite scaffold-materials. J. Mater. Chem. B 2015, 3, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ishikawa, K. Effects of nanopores on the mechanical strength, osteoclastogenesis, and osteogenesis in honeycomb scaffolds. J. Mater. Chem. B 2020, 8, 8536–8545. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, M.A.; Yilgör, E.; Yilgör, I. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr. Polym. 2017, 175, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.J.; Lin, F.H.; Chen, K.S.; Sun, J.S. Thermal decomposition and reconstitution of hydroxyapatite in air atmosphere. Biomaterials 1999, 20, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Malla, K.P.; Regmi, S.; Nepal, A.; Bhattarai, S.; Yadav, R.J.; Sakurai, S.; Adhikari, R. Extraction and Characterization of Novel Natural Hydroxyapatite Bioceramic by Thermal Decomposition of Waste Ostrich Bone. Int. J. Biomater. 2020, 2020, 1690178. [Google Scholar] [CrossRef] [PubMed]
- Natasha, A.N.; Singh, R.; Bin Abd Shukor, M.H.; Young, T.C.; Purbolaksono, J.; Sopyan, I.; Toulouei, R. Synthesis and Properties of Biphasic Calcium Phosphate Prepared by Different Methods. Adv. Mater. Res. 2014, 970, 20–25. [Google Scholar] [CrossRef]
- McDermott, A.M.; Mason, D.E.; Lin, A.S.P.; Guldberg, R.E.; Boerckel, J.D. Influence of structural load-bearing scaffolds on mechanical load- and BMP-2-mediated bone regeneration. J. Mech. Behav. Biomed. Mater. 2016, 62, 169–181. [Google Scholar] [CrossRef]
- Kumar, B.Y.S.; Isloor, A.M.; Kumar, G.C.M.; Inamuddin; Asiri, A.M. Nanohydroxyapatite Reinforced Chitosan Composite Hydrogel with Tunable Mechanical and Biological Properties for Cartilage Regeneration. Sci. Rep. 2019, 9, 15957. [Google Scholar] [CrossRef]
- Le, H.R.; Qu, S.; Mackay, R.E.; Rothwell, R. Fabrication and mechanical properties of chitosan composite membrane containing hydroxyapatite particles. J. Adv. Ceram. 2012, 1, 66–71. [Google Scholar] [CrossRef]
- Adamski, R.; Siuta, D. Mechanical, Structural, and Biological Properties of Chitosan/Hydroxyapatite/Silica Composites for Bone Tissue Engineering. Molecules 2021, 26, 1976. [Google Scholar] [CrossRef] [PubMed]










| Scaffold | |
|---|---|
| AQ | 0.51 ± 0.18 |
| B | 0.71 ± 0.07 |
| H | 0.70 ± 0.14 |
| BH | 0.65 ± 0.09 |
| Scaffold | R (Å) | (ns) | (%) | Vp (Å3) |
|---|---|---|---|---|
| AQ | 2.7 ± 0.1 | 1.85 ± 0.02 | 12.2 ± 0.1 | 82.4 ± 9.1 |
| B | 2.7 ± 0.1 | 1.85 ± 0.02 | 11.3 ± 0.1 | 82.4 ± 9.1 |
| H | 2.5 ± 0.1 | 1.69 ± 0.02 | 14.3 ± 0.1 | 65.4 ± 7.9 |
| BH | 2.4 ± 0.1 | 1.59 ± 0.02 | 15.7 ± 0.1 | 57.9 ± 7.2 |
| Scaffolds | Td (°C) | Mass Loss (%) | Final Residue (%) | ||||
|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 1 | Stage 2 | Stage 3 | ||
| AQ | 38.19 | 234.97 | |||||
| 130.79 | 269.02 | - | 18.76 | 33.11 | - | 23.82 | |
| 297.62 | |||||||
| B | 38.87 | 278.55 | 429.71 | 6.96 | 22.33 | 13.24 | 56.49 |
| H | 39.55 | 209.10 | 436.52 | 6.15 | 30.17 | 17.10 | 43.45 |
| 262.21 | |||||||
| BH | 42.96 | 260.85 | 431.08 | 6.65 | 24.04 | 17.85 | 49.21 |
| Scaffolds | Young’s Modulus (kPa) | Compressive Stress after 25% Strain (kPa) |
|---|---|---|
| AQ | 59.0 ± 2.9 | 15.8 ± 2.6 |
| B | 41.9 ± 1.9 | 10.7 ± 1.5 |
| H | 64.6 ± 5.6 | 7.8 ± 1.0 |
| BH | 52.0 ± 8.8 | 7.8 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, R.; Gordo, P.M.; Costa, B.F.O.; Alves, P. Formulation and Characterization of Chitosan-Based Mixed-Matrix Scaffold for Tissue Engineering. Macromol 2024, 4, 253-268. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol4020014
Lopes R, Gordo PM, Costa BFO, Alves P. Formulation and Characterization of Chitosan-Based Mixed-Matrix Scaffold for Tissue Engineering. Macromol. 2024; 4(2):253-268. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol4020014
Chicago/Turabian StyleLopes, Rita, Paulo M. Gordo, Benilde F. O. Costa, and Patrícia Alves. 2024. "Formulation and Characterization of Chitosan-Based Mixed-Matrix Scaffold for Tissue Engineering" Macromol 4, no. 2: 253-268. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol4020014