Predicting Coordination Variability of Selected Lower Extremity Couplings during a Cutting Movement: An Investigation of Deep Neural Networks with the LSTM Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
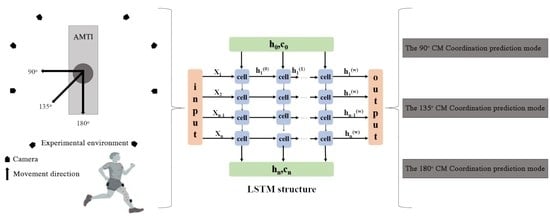
2.3. Procedures
2.4. Data Processing
2.4.1. Initial Data Processing
2.4.2. A Quantitative Approach to Coordination
2.5. Long Short Term Memory (LSTM) Network Algorithm Model
2.6. Statistical Analysis
3. Results
3.1. Shapiro-Wilk
3.2. SnPM1d
3.3. Performance of LSTM Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Brughelli, M.; Cronin, J.; Levin, G.; Chaouachi, A. Understanding Change of Direction Ability in Sport. Sports Med. 2008, 38, 1045–1063. [Google Scholar] [CrossRef] [PubMed]
- Besier, T.F.; Lloyd, D.G.; Ackland, T.R.; Cochrane, J.L. Anticipatory effects on knee joint loading during running and cutting maneuvers. Med. Sci. Sports Exerc. 2001, 33, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Hader, K.; Mendez-Villanueva, A.; Ahmaidi, S.; Williams, B.K.; Buchheit, M. Changes of direction during high-intensity intermittent runs: Neuromuscular and metabolic responses. BMC Sports Sci. Med. Rehabil. 2014, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Bloomfield, J.; Polman, R.; O’ Donoghue, P. Physical demands of different positions in FA Premier League soccer. J. Sports Sci. Med. 2007, 6, 63. [Google Scholar] [PubMed]
- Dos’ Santos, T.; McBurnie, A.; Comfort, P.; Jones, P.A. The Effects of Six-Weeks Change of Direction Speed and Technique Modification Training on Cutting Performance and Movement Quality in Male Youth Soccer Players. Sports 2019, 7, 205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Lu, Q. A Current Review of Foot Disorder and Plantar Pressure Alternation in the Elderly. Phys. Act. Health 2020, 4, 95–106. [Google Scholar] [CrossRef]
- Reilly, T.; Williams, A.M.; Nevill, A.; Franks, A. A multidisciplinary approach to talent identification in soccer. J. Sports Sci. 2000, 18, 695–702. [Google Scholar] [CrossRef]
- David, S.; Mundt, M.; Komnik, I.; Potthast, W. Understanding cutting maneuvers—The mechanical consequence of preparatory strategies and foot strike pattern. Hum. Mov. Sci. 2018, 62, 202–210. [Google Scholar] [CrossRef]
- Schreurs, M.J.; Benjaminse, A.; Lemmink, K.A. Sharper angle, higher risk? The effect of cutting angle on knee mechanics in invasion sport athletes. J. Biomech. 2017, 63, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Garrick, J.G. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am. J. Sports Med. 1977, 5, 241–242. [Google Scholar] [CrossRef]
- Nyland, J.A.; Shapiro, R.; Caborn, D.N.; Nitz, A.J.; Malone, T.R. The effect of quadriceps femoris, hamstring, and placebo eccentric fatigue on knee and ankle dynamics during crossover cutting. J. Orthop. Sports Phys. Ther. 1997, 25, 171–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, S.; Komnik, I.; Peters, M.; Funken, J.; Potthast, W. Identification and risk estimation of movement strategies during cutting maneuvers. J. Sci. Med. Sport 2017, 20, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.G.; Huang, X.; Van Den Bogert, A.J. Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: Implications for ACL injury. Clin. Biomech. 2005, 20, 863–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanrenterghem, J.; Venables, E.; Pataky, T.; Robinson, M.A. The effect of running speed on knee mechanical loading in females during side cutting. J. Biomech. 2012, 45, 2444–2449. [Google Scholar] [CrossRef]
- Miller, P.; Brinkmann, D.J.; Ramsenthaler, C.; Gollhofer, A.; Gehring, D. Mind your step: Predicting maximum ankle inversion during cutting movements in soccer. Sports Biomech. 2021, 1–15, in press. [Google Scholar] [CrossRef]
- Shimokochi, Y.; Ide, D.; Kokubu, M.; Nakaoji, T. Relationships Among Performance of Lateral Cutting Maneuver From Lateral Sliding and Hip Extension and Abduction Motions, Ground Reaction Force, and Body Center of Mass Height. J. Strength Cond. Res. 2013, 27, 1851–1860. [Google Scholar] [CrossRef]
- Rodrigues, G.; Dias, A.; Ribeiro, D.; Bertoncello, D. Relationship Between Isometric Hip Torque With Three Kinematic Tests in Soccer Players. Phys. Act. Health 2020, 4, 142–149. [Google Scholar] [CrossRef]
- Khayambashi, K.; Mohammadkhani, Z.; Ghaznavi, K.; Lyle, M.A.; Powers, C.M. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and hip strength in females with patellofemoral pain: A randomized controlled trial. J. Orthop. Sports Phys. Ther. 2012, 42, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Havens, K.L.; Sigward, S.M. Whole body mechanics differ among running and cutting maneuvers in skilled athletes. Gait Posture 2015, 42, 240–245. [Google Scholar] [CrossRef]
- Scholz, J.P. Dynamic pattern theory—Some implications for therapeutics. Phys. Ther. 1990, 70, 827–843. [Google Scholar] [CrossRef]
- Harbourne, R.T.; Stergiou, N. Movement variability and the use of nonlinear tools: Principles to guide physical therapist practice. Phys. Ther. 2009, 89, 267–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamill, J.; van Emmerik, R.E.; Heiderscheit, B.C.; Li, L. A dynamical systems approach to lower extremity running injuries. Clin. Biomech. 1999, 14, 297–308. [Google Scholar] [CrossRef]
- Crowther, R.G.; Spinks, W.L.; Leicht, A.S.; Quigley, F.; Golledge, J. Intralimb coordination variability in peripheral arterial disease. Clin. Biomech. 2008, 23, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, C.; Bloo, J.; Kooloos, J.; Van Kampen, A.; De Witte, J.; Wagenaar, R. Coordination and stability of one-legged hopping patterns in patients with anterior cruciate ligament reconstruction: Preliminary results. Clin. Biomech. 2003, 18, 84–87. [Google Scholar] [CrossRef]
- Pope, J.P.; Pelletier, L.G.; Guertin, C. Examining the role ones’ stage of change plays in understanding the relationship between motivation and physical activity. Phys. Act. Health 2021, 5, 120–132. [Google Scholar] [CrossRef]
- Di Paolo, S.; Zaffagnini, S.; Pizza, N.; Grassi, A.; Bragonzoni, L. Poor Motor Coordination Elicits Altered Lower Limb Biomechanics in Young Football (Soccer) Players: Implications for Injury Prevention through Wearable Sensors. Sensors 2021, 21, 4371. [Google Scholar] [CrossRef]
- Hamill, J.; Palmer, C.; Van Emmerik, R.E.A. Coordinative variability and overuse injury. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2012, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Tiberio, D. The effect of excessive subtalar joint pronation on patellofemoral mechanics: A theoretical model. J. Orthop. Sports Phys. Ther. 1987, 9, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.W.; Yen, H.C.; Chen, H.L. Comparisons of the inter-joint coordination between leading and trailing limbs when crossing obstacles of different heights. Gait Posture 2008, 27, 309–315. [Google Scholar] [CrossRef]
- Davids, K.; Bennett, S.; Newell, K.M. Movement System Variability; Human kinetics: Champaign, IL, USA, 2006. [Google Scholar]
- Patoz, A.; Lussiana, T.; Breine, B.; Gindre, C.; Malatesta, D. Both a single sacral marker and the whole-body center of mass accurately estimate peak vertical ground reaction force in running. Gait Posture 2021, 89, 186–192. [Google Scholar] [CrossRef]
- Verheul, J.; Nedergaard, N.J.; Vanrenterghem, J.; Robinson, M.A. Measuring biomechanical loads in team sports–from lab to field. Sci. Med. Footb. 2020, 4, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Van der Kruk, E.; Reijne, M.M. Accuracy of human motion capture systems for sport applications; state-of-the-art review. Eur. J. Sport Sci. 2018, 18, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, S.; Lopomo, N.F.; Della Villa, F.; Paolini, G.; Figari, G.; Bragonzoni, L.; Grassi, A.; Zaffagnini, S. Rehabilitation and return to sport assessment after anterior cruciate ligament injury: Quantifying joint kinematics during complex high-speed tasks through wearable sensors. Sensors 2021, 21, 2331. [Google Scholar] [CrossRef] [PubMed]
- Camomilla, V.; Bergamini, E.; Fantozzi, S.; Vannozzi, G. Trends Supporting the In-Field Use of Wearable Inertial Sensors for Sport Performance Evaluation: A Systematic Review. Sensors 2018, 18, 873. [Google Scholar] [CrossRef] [Green Version]
- Camomilla, V.; Dumas, R.; Cappozzo, A. Human movement analysis: The soft tissue artefact issue. J. Biomech. 2017, 62, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Mei, Q.; Wang, A.; Shim, V.; Fernandez, J.; Gu, Y. Evaluating function in the hallux valgus foot following a 12-week minimalist footwear intervention: A pilot computational analysis. J Biomech. 2022, 132, 110941. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mitchell, E.; Richter, C.; Destelle, F.; Gowing, M.; O’Connor, N.E.; Moran, K. Toward automatic activity classification and movement assessment during a sports training session. IEEE Internet Things J. 2014, 2, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, E.; Monaghan, D.; O’ Connor, N.E. Classification of sporting activities using smartphone accelerometers. Sensors 2013, 13, 5317–5337. [Google Scholar] [CrossRef] [Green Version]
- Kobsar, D.; Osis, S.T.; Hettinga, B.A.; Ferber, R. Classification accuracy of a single tri-axial accelerometer for training background and experience level in runners. J. Biomech. 2014, 47, 2508–2511. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y. Application of supervised machine learning algorithms in the classification of sagittal gait patterns of cerebral palsy children with spastic diplegia. Comput. Biol. Med. 2019, 106, 33–39. [Google Scholar] [CrossRef]
- Zago, M.; Sforza, C.; Dolci, C.; Tarabini, M.; Galli, M. Use of machine learning and wearable sensors to predict energetics and kinematics of cutting maneuvers. Sensors 2019, 19, 3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Merwe, C.; Shultz, S.P.; Colborne, G.R.; Hébert-Losier, K.; Fink, P.W. Using a modified vector coding technique to describe the calcaneus-shank coupling relationship during unanticipated changes of direction: Theoretical implications for prophylactic ACL strategies. Sports Biomech. 2022, 1–21, in press. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.; van Emmerik, R.; Jewell, C.; Hamill, J. Coordination and variability during anticipated and unanticipated sidestepping. Gait Posture 2019, 67, 1–8. [Google Scholar] [CrossRef]
- Delp, S.L.; Loan, J.P.; Hoy, M.G.; Zajac, F.E.; Topp, E.L.; Rosen, J.M. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans. Biomed. Eng. 1990, 37, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Forner-Cordero, A.; Mateu-Arce, M.; Forner-Cordero, I.; Alcántara, E.; Moreno, J.; Pons, J.L. Study of the motion artefacts of skin-mounted inertial sensors under different attachment conditions. Physiol. Meas. 2008, 29, N21. [Google Scholar] [CrossRef]
- Wilson, C.; Simpson, S.E.; Van Emmerik, R.E.; Hamill, J. Coordination variability and skill development in expert triple jumpers. Sports Biomech. 2008, 7, 2–9. [Google Scholar] [CrossRef]
- Heiderscheit, B.C.; Hamill, J.; van Emmerik, R.E. Variability of stride characteristics and joint coordination among individuals with unilateral patellofemoral pain. J. Appl. Biomech. 2002, 18, 110–121. [Google Scholar] [CrossRef]
- Samaan, M.A.; Hoch, M.C.; Ringleb, S.I.; Bawab, S.; Weinhandl, J.T. Isolated hamstrings fatigue alters hip and knee joint coordination during a cutting maneuver. J. Appl. Biomech. 2015, 31, 102–110. [Google Scholar] [CrossRef]
- Jayashree, P.; Ragupathy, U.S. Segmentation of cartilage in knee magnetic resonance images using Gabor and matched filter and classification of osteoarthritis using adaptive neuro-fuzzy inference system. Int. J. Biomed. Eng. Technol. 2021, 37, 290–307. [Google Scholar] [CrossRef]
- Yu, Y.; Si, X.; Hu, C.; Zhang, J. A review of recurrent neural networks: LSTM cells and network architectures. Neural Comput. 2019, 31, 1235–1270. [Google Scholar] [CrossRef]
- Hamill, J.; Haddad, J.M.; McDermott, W.J. Issues in quantifying variability from a dynamical systems perspective. J. Appl. Biomech. 2000, 16, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Haghighat, F.; Rezaie, M.; Ebrahimi, S.; Shokouhyan, S.M.; Motealleh, A.; Parnianpour, M. Coordination variability during walking and running in individuals with and without patellofemoral pain Part 1: Lower limb intersegmental coordination variability. J. Med. Biol. Eng. 2021, 41, 295–304. [Google Scholar] [CrossRef]
- Pollard, C.D.; Heiderscheit, B.C.; Van Emmerik, R.E.; Hamill, J. Gender differences in lower extremity coupling variability during an unanticipated cutting maneuver. J. Appl. Biomech. 2005, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepavac, D.; Field-Fote, E.C. Vector coding: A technique for quantification of intersegmental coupling in multicyclic behaviors. J. Appl. Biomech. 2001, 17, 259–270. [Google Scholar] [CrossRef]
- Mao, S.; Sejdić, E. A Review of Recurrent Neural Network-Based Methods in Computational Physiology. IEEE Trans. Neural Netw. Learn. Syst. 2022, 1–21, in press. [Google Scholar] [CrossRef]
- Hopfield, J.J. Neural networks and physical systems with emergent collective computational abilities. Proc. Natl. Acad. Sci. USA 1982, 79, 2554–2558. [Google Scholar] [CrossRef] [Green Version]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Preece, S.J.; Goulermas, J.Y.; Kenney, L.P.; Howard, D.; Meijer, K.; Crompton, R. Activity identification using body-mounted sensors—A review of classification techniques. Physiol. Meas. 2009, 30, R1. [Google Scholar] [CrossRef]
- Kadaba, M.; Ramakrishnan, H.; Wootten, M.; Gainey, J.; Gorton, G.; Cochran, G. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J. Orthop. Res. 1989, 7, 849–860. [Google Scholar] [CrossRef]
- Shuai, Z.; Dong, A.; Liu, H.; Cui, Y. Reliability and Validity of an Inertial Measurement System to Quantify Lower Extremity Joint Angle in Functional Movements. Sensors 2022, 22, 863. [Google Scholar] [CrossRef]
- Pataky, T.C.; Vanrenterghem, J.; Robinson, M.A. Zero-vs. one-dimensional, parametric vs. non-parametric, and confidence interval vs. hypothesis testing procedures in one-dimensional biomechanical trajectory analysis. J. Biomech. 2015, 48, 1277–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, P.; Schütte, K.; Vanwanseele, B.; Jacobs, J.; Dennerlein, J.; Schiffman, J.; Fournier, P.; Hu, B. Machine learning algorithms can classify outdoor terrain types during running using accelerometry data. Gait Posture 2019, 74, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wang, A.; Gu, Y.; Zhao, L.; Shim, V.; Fernandez, J. Recent Machine Learning Progress in Lower Limb Running Biomechanics With Wearable Technology: A Systematic Review. Front. Neurorobotics 2022, 16, 913052. [Google Scholar] [CrossRef] [PubMed]
- Rapp, E.; Shin, S.; Thomsen, W.; Ferber, R.; Halilaj, E. Estimation of kinematics from inertial measurement units using a combined deep learning and optimization framework. J. Biomech. 2021, 116, 110229. [Google Scholar] [CrossRef]
- Xu, D.; Quan, W.; Zhou, H.; Sun, D.; Baker, J.S.; Gu, Y. Explaining the differences of gait patterns between high and low-mileage runners with machine learning. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Picerno, P. 25 years of lower limb joint kinematics by using inertial and magnetic sensors: A review of methodological approaches. Gait Posture 2017, 51, 239–246. [Google Scholar] [CrossRef]
- Zucchini, W. An introduction to model selection. J. Math. Psychol. 2000, 44, 41–61. [Google Scholar] [CrossRef]
- Jaderberg, M.; Simonyan, K.; Vedaldi, A.; Zisserman, A. Synthetic data and artificial neural networks for natural scene text recognition. arXiv 2014, arXiv:1406.2227. [Google Scholar]
- Ordóñez, F.J.; Roggen, D. Deep convolutional and lstm recurrent neural networks for multimodal wearable activity recognition. Sensors 2016, 16, 115. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk, P.G.; Roland, M.; Hahn, M.E. Sensitivity of biomechanical outcomes to independent variations of hindfoot and forefoot stiffness in foot prostheses. Hum. Mov. Sci. 2017, 54, 154–171. [Google Scholar] [CrossRef]
- Dempster, J.; Dutheil, F.; Ugbolue, U.C. The prevalence of lower extremity injuries in running and associated risk factors: A systematic review. Phys. Act. Health 2021, 5, 133–145. [Google Scholar] [CrossRef]
- Shao, E.; Lu, Z.; Cen, X.; Zheng, Z.; Sun, D.; Gu, Y. The Effect of Fatigue on Lower Limb Joint Stiffness at Different Walking Speeds. Diagnostics 2022, 12, 1470. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.G.; Goldberg, E.J. The effect of lower extremity selective voluntary motor control on interjoint coordination during gait in children with spastic diplegic cerebral palsy. Gait Posture 2009, 29, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Latash, M.L.; Scholz, J.P.; Schöner, G. Toward a new theory of motor synergies. Mot. Control. 2007, 11, 276–308. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.W.; Stokic, D.S. Intersegmental coordination of gait after hemorrhagic stroke. Exp. Brain Res. 2015, 233, 125–135. [Google Scholar] [CrossRef]
- Heiderscheit, B.C. Movement variability as a clinical measure for locomotion. J. Appl. Biomech. 2000, 16, 419–427. [Google Scholar] [CrossRef]
- Imwalle, L.E.; Myer, G.D.; Ford, K.R.; Hewett, T.E. Relationship between hip and knee kinematics in athletic women during cutting maneuvers: A possible link to noncontact anterior cruciate ligament injury and prevention. J. Strength Cond. Res. /Natl. Strength Cond. Assoc. 2009, 23, 2223. [Google Scholar] [CrossRef] [Green Version]
- Arutyunyan, G.; Gurfinkel, V.; Mirskii, M. Organization of movements on execution by man of an exact postural task. Biophysics 1969, 14, 1162–1167. [Google Scholar]
- Yahya, U.; Senanayake, S.M.N.; Naim, A.G. Characterising leg-dominance in healthy netballers using 3D kinematics-electromyography features’ integration and machine learning techniques. Int. J. Biomed. Eng. Technol. 2022, 39, 65–92. [Google Scholar] [CrossRef]







| CM Direction (°) | Significance (p) | |
|---|---|---|
| 90 | 0.012748 | |
| Thigh A/A-Leg F/E | 135 | 0.000870 |
| 180 | 0.000436 | |
| 90 | 0.000119 | |
| Hip R-Knee F/E | 135 | 0.000002 |
| 180 | 0.000003 | |
| 90 | 0.007998 | |
| Knee F/E-Ankle R | 135 | 0.000006 |
| 180 | 0.000227 | |
| 90 | 0.000960 | |
| Vertical ground reaction force | 135 | 0.000756 |
| 180 | 0.000327 |
| The Couplings | Direction (°) | Mean (SD) | Max (SD) | Post Hoc Test | |
|---|---|---|---|---|---|
| Thigh A/A-Leg F/E | 90 | 0.564 (0.019) | 0.690 (0.014) | p < 0.001 (68–100% stride) | p < 0.001 (72–100% stride) |
| 135 | 0.588 (0.012) | 0.699 (0.007) | — | p > 0.05 | |
| 180 | 0.575 (0.012) | 0.697 (0.007) | — | — | |
| Hip R-Knee F/E | 90 | 0.554 (0.020) | 0.688 (0.008) | p < 0.001 (69–91% stride) | p < 0.001 (56–63% stride; 75–100% stride) |
| 135 | 0.575 (0.012) | 0.697 (0.010) | — | p > 0.05 | |
| 180 | 0.575 (0.014) | 0.697 (0.020) | — | — | |
| Knee F/E-Ankle R | 90 | 0.543 (0.020) | 0.685 (0.008) | p < 0.001 (72–100% stride) | p < 0.001 (77–100% stride) |
| 135 | 0.566 (0.016) | 0.698 (0.006) | — | p > 0.05 | |
| 180 | 0.555 (0.016) | 0.695 (0.017) | — | — | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, E.; Mei, Q.; Ye, J.; Ugbolue, U.C.; Chen, C.; Gu, Y. Predicting Coordination Variability of Selected Lower Extremity Couplings during a Cutting Movement: An Investigation of Deep Neural Networks with the LSTM Structure. Bioengineering 2022, 9, 411. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9090411
Shao E, Mei Q, Ye J, Ugbolue UC, Chen C, Gu Y. Predicting Coordination Variability of Selected Lower Extremity Couplings during a Cutting Movement: An Investigation of Deep Neural Networks with the LSTM Structure. Bioengineering. 2022; 9(9):411. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9090411
Chicago/Turabian StyleShao, Enze, Qichang Mei, Jingyi Ye, Ukadike C. Ugbolue, Chaoyi Chen, and Yaodong Gu. 2022. "Predicting Coordination Variability of Selected Lower Extremity Couplings during a Cutting Movement: An Investigation of Deep Neural Networks with the LSTM Structure" Bioengineering 9, no. 9: 411. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9090411