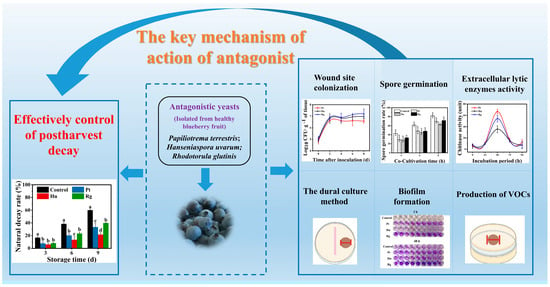
Inhibitory Effect and Potential Antagonistic Mechanism of Isolated Epiphytic Yeasts against Botrytis cinerea and Alternaria alternata in Postharvest Blueberry Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruits
2.2. Pathogen Isolation
2.3. Yeast Preparation
2.4. Biocontrol Effect of the Three Yeasts on B. cinerea and A. alternata in Blueberry Fruits
2.5. Antagonistic Activity on Agar Plates
2.6. Impact of Yeasts on B. cinerea and A. alternata Spore Germination In Vitro
2.7. Effects of VOCs In Vitro
2.8. Biofilm Formation
2.9. Extracellular Lytic Enzymes Activities
2.10. Colonization of Fruit Wounds
2.11. The Impact of Yeasts on the Natural Infection and the Quality of Blueberry Fruits
2.12. Statistical Analysis
3. Results
3.1. Biocontrol Efficacy of Yeasts in B. cinerea and A. alternata In Vivo
3.2. Function of Yeasts in B. cinerea and A. alternata Mycelial Growth In Vitro
3.3. Function of Yeasts in B. cinerea and A. alternata Spore Germination In Vitro
3.4. The Antagonistic and Inhibitory Activities of VOCs In Vitro
3.5. Biofilm-Forming Capacity
3.6. Extracellular Lytic Enzyme Activity
3.7. Wound Site Colonization
3.8. Effects of H. uvarum, P. terrestris, and R. glutinis on Natural Disease Incidence and Quality of Blueberry Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular mechanism and health role of functional ingredients in blueberry for chronic disease in human beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef] [PubMed]
- Sivapragasam, N.; Neelakandan, N.; Rupasinghe, H.P.V. Potential health benefits of fermented blueberry: A review of current scientific evidence. Trends Food Sci. Technol. 2023, 132, 103–120. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Tran, T.T.D. Blueberry supplementation in neuronal health and protective technologies for efficient delivery of blueberry anthocyanins. Biomolecules 2021, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Bell, S.R.; Hernández Montiel, L.G.; González Estrada, R.R.; Gutiérrez Martínez, P. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT-Food Sci. Technol. 2021, 149, 112046. [Google Scholar] [CrossRef]
- Umagiliyage, A.L.; Becerra-Mora, N.; Kohli, P.; Fisher, D.J.; Choudhary, R. Antimicrobial efficacy of liposomes containing D-limonene and its effect on the storage life of blueberries. Postharvest Biol. Technol. 2017, 128, 130–137. [Google Scholar] [CrossRef]
- Ding, J.; Liu, C.; Huang, P.; Zhang, Y.; Hu, X.; Li, H.; Liu, Y.; Chen, L.; Liu, Y.; Qin, W. Effects of thymol concentration on postharvest diseases and quality of blueberry fruit. Food Chem. 2023, 402, 134227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Yang, Q.; Apaliya, M.T. Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Q.; Zhao, Q.; Dhanasekaran, S.; Ahima, J.; Zhang, X.; Zhou, S.; Droby, S.; Zhang, H. Aureobasidium pullulans S-2 reduced the disease incidence of tomato by influencing the postharvest microbiome during storage. Postharvest Biol. Technol. 2022, 185, 111809. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Q.; Fang, T.; Shen, Y.; Jing, S.; Guo, N. Glycine enhances oxidative stress tolerance and biocontrol efficacy of Sporidiobolus pararoseus against Aspergillus niger decay of apples. Foods 2023, 12, 4121. [Google Scholar] [CrossRef]
- Sui, Y.; Wang, Z.; Zhang, D.; Wang, Q. Oxidative stress adaptation of the antagonistic yeast, Debaryomyces hansenii, increases fitness in the microenvironment of kiwifruit wound and biocontrol efficacy against postharvest diseases. Biol. Control 2021, 152, 104428. [Google Scholar] [CrossRef]
- López-Cruz, R.; Segarra, G.; Torres, R.; Teixidó, N.; Ragazzo-Sanchez, J.A.; Calderon-Santoyo, M. Biocontrol efficacy of Meyerozyma guilliermondii LMA-Cp01 against post-harvest pathogens of fruits. Arch. Phytopathol. Pflanzenschutz. 2023, 56, 1003–1020. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Xu, C.; Jiang, Z.; Liu, J.; Sui, Y.; Zhang, H. Control of postharvest blue and gray mold in kiwifruit by Wickerhamomyces anomalus and its mechanism of antifungal activity. Postharvest Biol. Technol. 2023, 201, 112345. [Google Scholar] [CrossRef]
- Ahmad, T.; Xing, F.; Nie, C.; Cao, C.; Xiao, Y.; Yu, X.; Moosa, A.; Liu, Y. Biocontrol potential of lipopeptides produced by the novel Bacillus subtilis strain Y17B against postharvest Alternaria fruit rot of cherry. Front Microbiol. 2023, 14, 1150217. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, D.; He, X.; Wang, F.; Wu, J.; Liu, Y.; Jiao, J.; Deng, J. Bacillus subtilis KLBC BS6 induces resistance and defence-related response against Botrytis cinerea in blueberry fruit. Physiol. Mol. Plant Pathol. 2021, 114, 101599. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, M.; Yang, Y.; Luo, K.; Liu, Z.; Liu, J.; Kuznetsova, N.; Liu, Z.; Sun, Q. Bacillus amyloliquefaciens M73 reduces postharvest decay and promotes anthocyanin accumulation in tarocco blood orange (Citrus sinensis (L.) Osbeck) during cold storage. Postharvest Biol. Technol. 2021, 182, 111698. [Google Scholar] [CrossRef]
- Ye, W.-Q.; Sun, Y.-F.; Tang, Y.-J.; Zhou, W.-W. Biocontrol potential of a broad-spectrum antifungal strain Bacillus amyloliquefaciens B4 for postharvest loquat fruit storage. Postharvest Biol. Technol. 2021, 174, 111439. [Google Scholar] [CrossRef]
- Bhan, C.; Asrey, R.; Singh, D.; Meena, N.K.; Vinod, B.R.; Menaka, M. Bioefficacy of bacteria and yeast bioagents on disease suppression and quality retention of stored kinnow mandarin fruits. Food Biosci. 2023, 53, 102743. [Google Scholar] [CrossRef]
- Li, H.; Jia, W.; Li, R.; Zhao, B.; Li, W.; Shao, Y. The combined application of Debaryomyces hansenii and Bacillus atrophaeus inhibits disease development and enhances postharvest quality in litchi fruit by activating flavonoid metabolism. Biol. Control 2023, 187, 105357. [Google Scholar] [CrossRef]
- Zong, Y.; Liu, J.; Li, B.; Qin, G.; Tian, S. Effects of yeast antagonists in combination with hot water treatment on postharvest diseases of tomato fruit. Biol. Control 2010, 54, 316–321. [Google Scholar] [CrossRef]
- Leng, J.; Yu, L.; Dai, Y.; Leng, Y.; Wang, C.; Chen, Z.; Wisniewski, M.; Wu, X.; Liu, J.; Sui, Y. Recent advances in research on biocontrol of postharvest fungal decay in apples. Crit. Rev. Food Sci. Nutr. 2023, 63, 10607–10620. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, S.; Deng, L.; Ming, J.; Zeng, K. Different mechanisms of action of isolated epiphytic yeasts against Penicillium digitatum and Penicillium italicum on citrus fruit. Postharvest Biol. Technol. 2019, 152, 100–110. [Google Scholar] [CrossRef]
- Oztekin, S.; Karbancioglu-Guler, F. Bioprospection of Metschnikowia sp. Isolates as biocontrol agents against postharvest fungal decays on lemons with their potential modes of action. Postharvest Biol. Technol. 2021, 181, 111634. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, L.; Li, S.; Gao, X.; Wu, N.; Zhao, Y.; Sun, W. Control of postharvest grey spot rot of loquat fruit with Metschnikowia pulcherrima E1 and potential mechanisms of action. Biol. Control 2021, 152, 104406. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Droby, S.; Preciado-Rangel, P.; Rivas-García, T.; González-Estrada, R.R.; Gutiérrez-Martínez, P.; Ávila-Quezada, G.D. A sustainable alternative for postharvest disease management and phytopathogens biocontrol in fruit: Antagonistic yeasts. Plants 2021, 10, 2641. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Peng, H.; Tian, S. Attachment capability of antagonistic yeast Rhodotorula glutinis to Botrytis cinerea contributes to biocontrol efficacy. Front. Microbiol. 2016, 7, 187725. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, L.; Zeng, K. Enhancement of biocontrol efficacy of Pichia membranaefaciens by hot water treatment in postharvest diseases of citrus fruit. Crop Prot. 2014, 63, 89–96. [Google Scholar] [CrossRef]
- Jin, Y.; Yip, H.; Samaranayake, Y.; Yau, J.; Samaranayake, L. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 2003, 41, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Saligkarias, I.; Gravanis, F.; Epton, H.A. Biological control of Botrytis cinerea on tomato plants by the use of epiphytic yeasts Candida guilliermondii strains 101 and US 7 and Candida oleophila strain I-182: II. a study on mode of action. Biol. Control 2002, 25, 151–161. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Zeng, K. Efficacy of Pichia membranaefaciens combined with chitosan against Colletotrichum gloeosporioides in citrus fruits and possible modes of action. Biol. Control 2016, 96, 39–47. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Zhou, X.; Wei, B.; Ji, S. The effect of ethylene absorbent treatment on the softening of blueberry fruit. Food Chem. 2018, 246, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, J.; Yan, H.; Liang, W.; Wang, G. Ethyl acetate produced by Hanseniaspora uvarum is a potential biocontrol agent against tomato fruit rot caused by Phytophthora nicotianae. Front. Microbiol. 2022, 13, 978920. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Miccoli, C.; Notardonato, I.; Avino, P.; Lima, G.; De Curtis, F.; Ianiri, G.; Castoria, R. Modulation of extracellular Penicillium expansum-driven acidification by Papiliotrema terrestris affects biosynthesis of patulin and has a possible role in biocontrol activity. Front. Microbiol. 2022, 13, 973670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Selection and evaluation of new antagonists for their efficacy against postharvest brown rot of peaches. Postharvest Biol. Technol. 2010, 55, 174–181. [Google Scholar] [CrossRef]
- Öztekin, S.; Karbancioglu-Guler, F. Biological control of green mould on mandarin fruit through the combined use of antagonistic yeasts. Biol. Control 2023, 180, 105186. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Banos, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Galván, A.I.; Hernández, A.; de Guía Córdoba, M.; Martín, A.; Serradilla, M.J.; López-Corrales, M.; Rodríguez, A. Control of toxigenic Aspergillus spp. In dried figs by volatile organic compounds (VOCs) from antagonistic yeasts. Int. J. Food Microbiol. 2022, 376, 109772. [Google Scholar] [CrossRef] [PubMed]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile organic compounds (VOCs) produced by biocontrol yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xiao, H.; Cheng, X.; Zhou, H.; Si, L. Hanseniaspora uvarum prolongs shelf life of strawberry via volatile production. Food Microbiol. 2017, 63, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dou, G.; Guo, H.; Zhang, Q.; Qin, X.; Yu, W.; Jiang, C.; Xiao, H. Volatile organic compounds of Hanseniaspora uvarum increase strawberry fruit flavor and defense during cold storage. Food Sci. Nutr. 2019, 7, 2625–2635. [Google Scholar] [CrossRef]
- Ajuna, H.B.; Lim, H.-I.; Moon, J.-H.; Won, S.-J.; Choub, V.; Choi, S.-I.; Yun, J.-Y.; Ahn, Y.S. The prospect of hydrolytic enzymes from Bacillus species in the biological control of pests and diseases in forest and fruit tree production. Int. J. Food Microbiol. 2023, 24, 16889. [Google Scholar] [CrossRef]
- de Oliveira, A.J.; Ono, M.A.; Suguiura, I.M.d.S.; Zucareli, C.; Garcia, E.B.; Olchanheski, L.R.; Ono, E.Y.S. Potential of yeasts as biocontrol agents against Fusarium graminearum in vitro and on corn. J. Appl. Microbiol. 2023, 134, lxad296. [Google Scholar] [CrossRef] [PubMed]
- Zamoum, M.; Allali, K.; Benadjila, A.; Zitouni, A.; Goudjal, Y. Formulation of biofungicides based on Streptomyces caeruleatus strain ZL-2 spores and efficacy against Rhizoctonia solani damping-off of tomato seedlings. Arch. Microbiol. 2022, 204, 629. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.N.; Kupper, K.C. Biofilm production by Aureobasidium pullulans improves biocontrol against sour rot in citrus. Food Microbiol. 2018, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Y.; Quan, S.; Qiu, J.-e.; Dhanasekaran, S.; Li, B.; Gu, X.; Zhang, X.; Zhang, H. Transcriptome analysis reveals mechanisms of the disease resistance in postharvest kiwifruit induced by Meyerozyma caribbica. Sci. Hortic. 2023, 322, 112452. [Google Scholar] [CrossRef]





| Isolates | Inhibition of Mycelial Growth of B. cinerea (%) | Inhibition of Mycelial Growth of A. alternata (%) | ||
|---|---|---|---|---|
| pH 4.5 | pH 6.0 | pH 4.5 | pH 6.0 | |
| P. terrestris | 45.79 ± 2.16 b | 37.01 ± 2.03 b | 38.29 ± 2.41 b | 30.14 ± 1.16 b |
| H. uvarum | 55.25 ± 1.72 a | 42.33 ± 1.44 a | 43.71 ± 1.07 a | 35.43 ± 1.32 a |
| R. glutinis | 31.85 ± 1.70 c | 26.67 ± 2.24 c | 26.73 ± 1.42 c | 17.64 ± 3.03 c |
| Isolates | Inhibition of Mycelial Growth of B. cinerea (%) | Inhibition of Mycelial Growth of A. alternata (%) | ||
|---|---|---|---|---|
| pH 4.5 | pH 6.0 | pH 4.5 | pH 6.0 | |
| P. terrestris | 36.01 ± 2.03 b | 28.66 ± 2.57 a | 24.36 ± 1.67 b | 19.65 ± 0.69 a |
| H. uvarum | 42.33 ± 1.44 a | 30.75 ± 1.28 a | 29.26 ± 1.93 a | 20.21 ± 3.42 a |
| R. glutinis | 21.67 ± 2.07 c | 20.85 ± 2.62 b | 18.54 ± 3.03 c | 12.64 ± 2.76 b |
| Storage Time (d) | Treatment | Decay Incidence (%) | Weight Loss (%) | Total Soluble Solid (%) | Titratable Acidity (%) | Vitamin C (mg per 100 g FW) |
|---|---|---|---|---|---|---|
| 0 | Control | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 8.43 ± 0.45 a | 0.40 ± 0.70 a | 95.40 ± 0.26 a |
| P. terrestris | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 8.77 ± 0.21 a | 0.40 ± 0.06 a | 95.66 ± 0.67 a | |
| H. uvarum | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 8.60 ± 0.15 a | 0.39 ± 0.06 a | 94.93 ± 0.66 a | |
| R. glutinis | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 8.63 ± 0.06 a | 0.41 ± 0.03 a | 95.27 ± 0.21 a | |
| 3 | Control | 16.67 ± 1.53 a | 13.86 ± 1.41 a | 9.87 ± 0.25 b | 0.53 ± 0.70 a | 88.30 ± 2.01 b |
| P. terrestris | 7.67 ± 2.52 b | 7.66 ± 0.95 b | 10.97 ± 0.70 a | 0.48 ± 0.03 a | 93.77 ± 0.71 a | |
| H. uvarum | 6.10 ± 1.55 b | 3.50 ± 0.31 c | 10.6 ± 0.20 ab | 0.26 ± 0.02 b | 95.07 ± 0.32 a | |
| R. glutinis | 8.10 ± 0.72 b | 2.79 ± 0.16 c | 11.1 ± 0.26 a | 0.31 ± 0.05 b | 93.57 ± 0.45 a | |
| 6 | Control | 38.33 ± 2.08 a | 17.47 ± 2.14 a | 7.83 ± 0.21 d | 0.35 ± 0.02 a | 86.57 ± 1.71 c |
| P. terrestris | 20.33 ± 2.52 b | 9.98 ± 2.86 b | 9.07 ± 0.11 c | 0.41 ± 0.04 ab | 91.83 ± 1.56 b | |
| H. uvarum | 13.33 ± 5.16 c | 5.31 ± 2.38 c | 9.53 ± 0.31 b | 0.24 ± 0.02 c | 94.37 ± 0.61 a | |
| R. glutinis | 23.33 ± 2.08 b | 4.30 ± 0.30 c | 10.2 ± 0.10 a | 0.28 ± 0.06 bc | 93.02 ± 0.66 a | |
| 9 | Control | 60.00 ± 1.00 a | 24.03 ± 3.19 a | 7.90 ± 0.36 c | 0.26 ± 0.01 a | 64.00 ± 2.40 c |
| P. terrestris | 33.33 ± 5.77 c | 18.55 ± 1.03 b | 9.03 ± 0.15 b | 0.21 ± 0.02 b | 77.73 ± 0.95 b | |
| H. uvarum | 21.67 ± 2.58 d | 14.31 ± 2.38 c | 9.53 ± 0.15 a | 0.15 ± 0.01 c | 88.89 ± 2.16 a | |
| R. glutinis | 39.67 ± 0.58 b | 13.03 ± 0.57 c | 8.23 ± 0.21 c | 0.15 ± 0.02 c | 78.56 ± 0.61 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, T.; Yuan, F.; Lv, X.; Zhou, Y. Inhibitory Effect and Potential Antagonistic Mechanism of Isolated Epiphytic Yeasts against Botrytis cinerea and Alternaria alternata in Postharvest Blueberry Fruits. Foods 2024, 13, 1334. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13091334
Li J, Yang T, Yuan F, Lv X, Zhou Y. Inhibitory Effect and Potential Antagonistic Mechanism of Isolated Epiphytic Yeasts against Botrytis cinerea and Alternaria alternata in Postharvest Blueberry Fruits. Foods. 2024; 13(9):1334. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13091334
Chicago/Turabian StyleLi, Jia, Ting Yang, Furong Yuan, Xinyue Lv, and Yahan Zhou. 2024. "Inhibitory Effect and Potential Antagonistic Mechanism of Isolated Epiphytic Yeasts against Botrytis cinerea and Alternaria alternata in Postharvest Blueberry Fruits" Foods 13, no. 9: 1334. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13091334