GC-IMS-Based Volatile Characteristic Analysis of Hypsizygus marmoreus Dried by Different Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. GC-IMS Analysis
2.3. Statistical Analysis
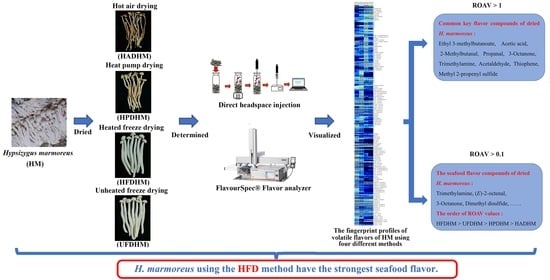
3. Results and Discussion
3.1. Differences in Volatile Compounds of H. marmoreus Dried by Different Methods
3.2. Qualitative Analysis of Volatile Compounds in H. marmoreus Dried by Different Methods
3.3. The Fingerprints of Volatile Compounds of H. marmoreus Dried by Different Methods
| NO. | Compound | Odor Description | Odor Threshold (μg/L) | ROAV | |||
|---|---|---|---|---|---|---|---|
| HAD | HPD | HFD | UFD | ||||
| 10, 11 | Ethyl 3-methylbutanoate | apple, pineapple, fruity | 0.00011 | 100.0000 | 100.0000 | 100.0000 | 100.0000 |
| 102, 103 | Acetic acid | sour, pungent, vinegar | 0.013 | 12.5090 | 13.4381 | 81.4056 | 41.4407 |
| 44 | 2-Methylbutanal | cocoa, almond | 0.001 | 1.7932 | 3.5902 | 37.9957 | 19.0935 |
| 41 | Propanal | alcohol, cocoa, nutty | 0.0048 | 3.8091 | 4.0892 | 27.7542 | 13.6690 |
| 113 | Methyl 2-propenyl sulfide | garlic, onion, alliaceous | 0.0005 | 3.1181 | 1.9411 | 26.7787 | 9.6130 |
| 114 | Trimethylamine | fishy, pungent | 0.02 | 1.6057 | 2.5491 | 24.2904 | 13.9812 |
| 78 | 3-Octanone | herbal, fresh, mushroom | 0.0013 | 2.3167 | 2.0636 | 21.2537 | 14.2491 |
| 43 | Acetaldehyde | whiskey, pungent, fruity | 0.0027 | 2.2393 | 1.8539 | 13.8660 | 7.0853 |
| 98, 99 | 2-Methylpropanoic acid | butter, strawberry, cheese | 0.04 | 1.9117 | 0.7959 | 12.8856 | 7.7170 |
| 95 | Thiophene | garlic, alliaceous | 0.0019 | 2.0711 | 1.3325 | 11.1635 | 7.3497 |
| 40 | Butanal | chocolate, herbaceous, floral, fruity | 0.002 | 0.4692 | 0.4184 | 5.6678 | 1.3346 |
| 29 | Heptanal | citrus, fatty, rancid | 0.003 | 0.3988 | 0.5889 | 3.9985 | 1.4950 |
| 27 | (E)-2-Octenal | nuts, green, fatty | 0.003 | 0.1910 | 0.3891 | 3.5683 | 1.2711 |
| 64 | 2-Methyl-1-propanol | solvent, ether, wine, bitter | 0.033 | 0.4660 | 0.3599 | 2.6215 | 1.7375 |
| 92 | 2-Pentylfuran | green beans, vegetable | 0.006 | 0.6026 | 0.4423 | 2.3472 | 1.3056 |
| 90 | Dimethyl disulfide | vegetable, nutty, meaty, green | 0.0084 | 0.1463 | 0.3206 | 2.1086 | 1.2490 |
| 39 | Acrolein | cherry, almond | 0.0083 | 0.1952 | 0.1764 | 2.0832 | 0.5202 |
| 91 | 2-Butylfuran | wine, sweet, fruity, spicy | 0.005 | 0.4275 | 0.3574 | 1.9303 | 1.3321 |
| 42 | 2-Methylpropanal | malt, pungent, green | 0.0015 | 1.2649 | 0.6996 | 1.6304 | 0.6312 |
| 65, 66 | 1-Propanol | fermented, fusel, pungent | 0.24 | 0.1407 | 0.1072 | 0.7133 | 0.4071 |
| 84 | 3-Nonanone | jasmin, herbal, fresh | 0.017 | 0.0284 | 0.0633 | 0.5642 | 0.3300 |
| 45 | cis-4-Heptenal | biscuit, dairy, green | 0.040 | 0.0043 | 0.0154 | 0.4871 | 0.3523 |
| 62, 63 | 1-Butanol | vanilla, fruit, balsam | 0.48 | 0.0322 | 0.0267 | 0.2572 | 0.1513 |
| 19 | Ethyl Acetate | pineapple, anise, fruity, green | 0.88 | 0.0343 | 0.0326 | 0.2430 | 0.1493 |
| 69 | Ethanol | ethereal, sweet | 0.62 | 0.0304 | 0.0300 | 0.2279 | 0.1478 |
| 8, 9 | Butyl acetate | sweet, banana | 0.13 | 0.0374 | 0.0410 | 0.2262 | 0.1452 |
| 37 | Pentanal | bready, berry, almond | 0.4 | 0.0168 | 0.0208 | 0.1783 | 0.0593 |
| 6, 7 | Isoamyl acetate | banana, fruity, sweet | 0.918 | 0.0337 | 0.0382 | 0.1616 | 0.1130 |
| 60, 61 | 3-Methyl-1-butanol | sweet, malty, rubber | 1.69 | 0.0220 | 0.0204 | 0.1523 | 0.0928 |
| 83 | Acetone | apple, pear, ethereal | 0.832 | 0.0262 | 0.0157 | 0.1043 | 0.0467 |
3.4. ROAV Analysis of Volatile Compounds in H. marmoreus Dried by Different Methods
3.5. Cluster Analysis of Volatile Compounds in H. marmoreus Dried by Different Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, C.; Duan, X.; Lin, L.; Wu, W.; Li, X.; Zeng, Z.; Luo, Q.; Liu, Y. A review on the edible mushroom as a source of special flavor: Flavor categories, influencing factors, and challenges. Food Front. 2023, 4, 1561–1577. [Google Scholar] [CrossRef]
- Lee, Y.; Jian, S.; Lian, P.; Mau, J. Antioxidant properties of extracts from a white mutant of the mushroom Hypsizigus marmoreus. J. Food Compos. Anal. 2008, 21, 116–124. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Shang, J.-J.; Li, Y.; Zhou, C.-L.; Hou, D.; Li, J.-L.; Tan, Q.; Bao, D.-P.; Yang, R.-H. The complete mitochondrial genome of the Basidiomycete edible fungus Hypsizygus marmoreus. Mitochondrial DNA Part B. 2018, 3, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, H.; Zhou, C.; Gong, M.; Li, Y.; Shao, Y.; Wu, Y.; Bao, D. Exogenous γ-aminobutyric acid (GABA) enhanced response to abiotic stress in Hypsizygus marmoreus by improving mycelial growth and antioxidant capacity. Metabolites 2024, 14, 94. [Google Scholar] [CrossRef]
- Li, J.; Lei, Y.; Qin, Z.; Liu, J.; Rensing, C.; Lin, Z.; Lin, D. Effects of seafood mushroom spent substrate solid-state fermentation combined with pgpr as a microbial fertilizer on the soil environment and growth promotion of Cenchrus fungigraminus. J. Soil Sci. Plant Nutr. 2024, 24, 1261–1277. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A. Development of flavor during drying and applications of edible mushrooms: A review. Dry. Technol. 2021, 39, 1685–1703. [Google Scholar] [CrossRef]
- Zheng, C.; Li, J.; Liu, H.; Wang, Y. Review of postharvest processing of edible wild-grown mushrooms. Food Res. Int. 2023, 173, 113223. [Google Scholar] [CrossRef]
- Deng, G.; Li, J.; Liu, H.; Wang, Y. Volatile compounds and aroma characteristics of mushrooms: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.; Lo, Y.M. Conventional and novel applications of edible mushrooms in today’s food industry. J. Food Process. Preserv. 2014, 38, 2146–2153. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, Y.; Shu, B.; Liu, Y.; Tu, X.; Ouyang, H.; Li, Y.; Tan, G.; Yu, Z.; Chen, F.; et al. Effects of four drying methods on Ganoderma lucidum volatile organic compounds analyzed via headspace solid-phase microextraction and comprehensive two-dimensional chromatography-time-of-flight mass spectrometry. Microchem. J. 2021, 166, 106258. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC–MS and electronic nose. LWT-Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, X.; Jia, M.; Dong, X.; Li, M.; Xu, H. Effect of different drying methods on the quality characteristics and non-volatile taste compounds of Hypsizygus marmoreus pileus and stipe. LWT-Food Sci. Technol. 2024, 192, 115685. [Google Scholar] [CrossRef]
- Wu, F.; Tang, J.; Pei, F.; Wang, S.; Chen, G.; Hu, Q.; Zhao, L. The influence of four drying methods on nonvolatile taste components of White Hypsizygus marmoreus. Eur. Food Res. Technol. 2015, 240, 823–830. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs. spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace—Gas chromatography–ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Rosales Martinez, A.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef]
- Gerhardt, N.; Birkenmeier, M.; Sanders, D.; Rohn, S.; Weller, P. Resolution-optimized headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) for non-targeted olive oil profiling. Anal. Bioanal. Chem. 2017, 409, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shen, S.; Huang, L.; Deng, G.; Wei, Y.; Ning, J.; Wang, Y. Revelation of volatile contributions in green teas with different aroma types by GC–MS and GC–IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef]
- Hou, H.; Liu, C.; Lu, X.; Fang, D.; Hu, Q.; Zhang, Y.; Zhao, L. Characterization of flavor frame in shiitake mushrooms (Lentinula edodes) detected by HS-GC-IMS coupled with electronic tongue and sensory analysis: Influence of drying techniques. LWT-Food Sci. Technol. 2021, 146, 111402. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC–MS. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2018, 1099, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Tang, B.; Li, Y.; Wu, L.; Weng, M.; Chen, J. Grey correlation analysis for physical and nutritional quality of Hypsizygus marmoreus from different drying methods. J. Nucl. Agric. Sci. 2021, 35, 2118–2126. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, P.; Zhang, W.; Zhu, H.; Wang, C.; Xie, N.; Wang, Y.; Pang, X.; Marie-Laure, F.; Lü, J.; et al. Free fatty acid hydrolyzed with lipases and their effects on enzyme-modified cheese flavor. Food Sci. Anim. Prod. 2023, 1, 9240031. [Google Scholar] [CrossRef]
- Huang, Q.; Dong, K.; Wang, Q.; Huang, X.; Wang, G.; An, F.; Luo, Z.; Luo, P. Changes in volatile flavor of yak meat during oxidation based on multi-omics. Food Chem. 2022, 371, 131103. [Google Scholar] [CrossRef]
- Liu, S.; Holland, R.; Crow, V. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Zamora, R.; Gallardo, E.; Hidalgo, F. Strecker degradation of phenylalanine initiated by 2,4-decadienal or methyl 13-oxooctadeca-9,11-dienoate in model systems. J. Agric. Food Chem. 2007, 55, 1308–1314. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Si, B.; Tong, A.; Lu, Y.; Lv, L. Synergistic inhibitory effect of multiple polyphenols from spice on acrolein during high-temperature processing. Foods 2023, 12, 2326. [Google Scholar] [CrossRef]
- Gomes, R.; Meek, M. World Health Organization & International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Li, B.; Zhang, T.; Dai, Y.; Jiang, G.; Peng, Y.; Wang, J.; Song, Y.; Ding, Z. Effects of probiotics on antioxidant activity, flavor compounds and sensory evaluation of Rosa roxburghii Tratt. LWT-Food Sci. Technol. 2023, 179, 114664. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Yao, F.; Gao, H. Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushrooms. J. Sci. Food Agric. 2019, 99, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Q.; Liu, Q.; Xia, X.; Wang, Y.; Kong, B. Effect of different types of smoking materials on the flavor, heterocyclic aromatic amines, and sensory property of smoked chicken drumsticks. Food Chem. 2022, 367, 130680. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X–20903555X. [Google Scholar] [CrossRef]
- Liu, Q.; Bau, T.; Jin, R.; Cui, X.; Zhang, Y.; Kong, W. Comparison of different drying techniques for shiitake mushroom (Lentinus edodes): Changes in volatile compounds, taste properties, and texture qualities. LWT-Food Sci. Technol. 2022, 164, 113651. [Google Scholar] [CrossRef]
- Cui, H.; Yu, J.; Zhai, Y.; Feng, L.; Chen, P.; Hayat, K.; Xu, Y.; Zhang, X.; Ho, C.-T. Formation and fate of Amadori rearrangement products in Maillard reaction. Trends Food Sci. Technol. 2021, 115, 391–408. [Google Scholar] [CrossRef]
- Yin, X.; Wei, Y.; Li, T.; Zhang, J.; Zou, L.; Cui, Q.; Lu, C.; Ning, J. Heterocyclic compounds formation in large-leaf yellow tea induced by the Maillard reaction at different roasting temperatures. LWT-Food Sci. Technol. 2023, 182, 114856. [Google Scholar] [CrossRef]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC × GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
- Huang, C.; Cui, H.; Hayat, K.; Zhang, X.; Ho, C. Variation of moisture state and taste characteristics during vacuum drying of Maillard reaction intermediates of hydrolyzed soybean protein and characterization of browning precursors via fluorescence spectroscopy. Food Res. Int. 2022, 162, 112086. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, C.; Peng, L.; Dong, Y.; Gao, Y.; Xu, J.; Ping, H.; Liu, S. Effect of vacuum roasting on total selenium content of selenium-enriched rapeseed, Maillard reaction products, oxidative stability and physicochemical properties of selenium-enriched rapeseed oil. Foods 2023, 12, 3204. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Wu, J.; Pan, M.; Ho, C. Reactivity and stability of selected flavor compounds. J. Food Drug Anal. 2015, 23, 176–190. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free. Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L.; Li, Z.; Wang, K.; Mao, D.; Jiang, J. Compilations of Odour Threshold Values in Air, Water and Other Media (Second Enlarged and Revised Edition); Science Press: Beijing, China, 2018. [Google Scholar]
- Murnane, S.; Lehocky, A.; Owens, P. Odor Thresholds for Chemicals with Established Occupational Health Standards, 2nd ed.; American Industrial Hygiene Association: Falls Church, VA, USA, 2013. [Google Scholar]
- Bi, J.; Lin, Z.; Li, Y.; Chen, F.; Liu, S.; Li, C. Effects of different cooking methods on volatile flavor compounds of chicken breast. J. Food Biochem. 2021, 45, e13770. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; He, J.; Zhou, Y.; Li, Y.; Zhou, H. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS-SPME-GC-MS, sensory evaluation, and chemometrics. Food Chem. 2022, 373, 131587. [Google Scholar] [CrossRef]
- Tang, B.; Wu, L.; Weng, M.; Chen, J.; Li, Y.; Lai, P. Effect of Hypsizygus marmoreus powder on cooking characteristics, color and texture of wheat noodles. Food Sci. Technol. 2022, 42, e00622. [Google Scholar] [CrossRef]
- Josephson, D. Seafood. In Volatile Compounds in Foods and Beverages; Routledge: New York, USA, 2017; pp. 179–202. [Google Scholar] [CrossRef]
- Wu, T.; Wang, M.; Wang, P.; Tian, H.; Zhan, P. Advances in the formation and control methods of undesirable flavors in fish. Foods 2022, 11, 2504. [Google Scholar] [CrossRef]
- Chen, G.; Wu, F.; Pei, F.; Cheng, S.; Muinde, B.; Hu, Q.; Zhao, L. Volatile components of white Hypsizygus marmoreus detected by electronic nose and HS-SPME-GC-MS: Influence of four drying methods. Int. J. Food Prop. 2017, 20, 2901–2910. [Google Scholar] [CrossRef]
- Han, Y.; Barringer, S. Formation of volatiles in the lipoxygenase pathway as affected by fruit type and temperature. J. Exp. Food Chem. 2015, 1, 1–7. [Google Scholar] [CrossRef]








| NO. | Volatile Compounds | RI | Rt (s) | Dt (RIP Rel) | Peak Intensity | |||
|---|---|---|---|---|---|---|---|---|
| HAD | HPD | HFD | UFD | |||||
| Esters | ||||||||
| 1 | Hexyl propionate | 1337.7 | 893.617 | 1.43424 | 241.61 ± 10.46 d | 464.64 ± 23.18 c | 757.47 ± 34.34 b | 828.52 ± 28.27 a |
| 2 | Hexyl acetate-M | 1282.4 | 793.596 | 1.3862 | 674.92 ± 99.27 a | 562.1 ± 48.77 a | 305.08 ± 29.93 b | 267.38 ± 4.71 b |
| 3 | Hexyl acetate-D | 1282.4 | 793.596 | 1.89535 | 156.32 ± 14.17 a | 113.69 ± 16.74 b | 60.86 ± 11.3 c | 57.72 ± 7.64 c |
| 4 | Pentyl acetate-M | 1185.3 | 643.622 | 1.31204 | 1150.78 ± 32.37 c | 1588.67 ± 12.9 a | 1413.03 ± 42.98 b | 1221.99 ± 19.45 c |
| 5 | Pentyl acetate-D | 1185.6 | 644.29 | 1.7582 | 451.8 ± 3.36 d | 1351.58 ± 44.56 a | 937.06 ± 85.42 b | 750.87 ± 3.7 c |
| 6 | Isoamyl acetate-M | 1135 | 544.741 | 1.30162 | 3365.42 ± 79.76 a | 2640.67 ± 133.59 b | 2286.55 ± 43.5 c | 2317.59 ± 15.86 c |
| 7 | Isoamyl acetate-D | 1135 | 544.741 | 1.74952 | 9328.41 ± 157.54 b | 11,216.06 ± 505.59 a | 5546.66 ± 277.33 d | 7923.42 ± 70.32 c |
| 8 | Butyl acetate-M | 1085.7 | 464.399 | 1.23694 | 1067.03 ± 51.34 a | 1059.32 ± 13.84 a | 873.34 ± 5.62 b | 935.29 ± 14.14 b |
| 9 | Butyl acetate-D | 1085.4 | 463.895 | 1.61559 | 926.87 ± 35.44 b | 1048.73 ± 77.18 a | 679.65 ± 26 c | 927.68 ± 5.24 b |
| 10 | Ethyl 3-methylbutanoate-M | 1078 | 453.832 | 1.26208 | 2174.67 ± 81.19 a | 1995 ± 24.28 b | 479.4 ± 45.81 d | 833.28 ± 10.68 c |
| 11 | Ethyl 3-methylbutanoate-D | 1079.1 | 455.342 | 1.64961 | 2336.09 ± 69.4 a | 2353.17 ± 178.56 a | 100.61 ± 21.15 b | 252.28 ± 3.87 b |
| 12 | Ethyl 2-methylbutanoate | 1063.8 | 435.215 | 1.64517 | 2387.04 ± 81.78 b | 2686.19 ± 211.72 a | 252.08 ± 32.34 d | 633.72 ± 10.77 c |
| 13 | 2-Methylpropyl acetate | 1025 | 387.918 | 1.60672 | 2759.58 ± 170.79 a | 1949.85 ± 151.1 b | 953.58 ± 74.75 c | 1954.3 ± 31.99 b |
| 14 | Methyl 2-methylbutanoate-M | 1008.9 | 369.804 | 1.21475 | 1014.19 ± 1.84 a | 943.87 ± 6.35 b | 499.46 ± 7.26 c | 364.92 ± 10.69 d |
| 15 | Methyl 2-methylbutanoate-D | 1009.4 | 370.307 | 1.51205 | 3729.79 ± 230.78 b | 2994.94 ± 78.08 c | 5011.2 ± 72.91 a | 3891.53 ± 102.22 b |
| 16 | Propyl acetate | 989.4 | 350.684 | 1.4736 | 8152.93 ± 87.35 a | 6641.38 ± 227.45 b | 2376.46 ± 106.77 d | 2844.48 ± 80.86 c |
| 17 | Ethyl isobutyrate | 976.5 | 340.62 | 1.55643 | 1752.69 ± 34.7 b | 2406.14 ± 105.73 a | 774.62 ± 171.94 d | 1260.66 ± 50.77 c |
| 18 | Ethyl propanoate | 969.9 | 335.589 | 1.44549 | 1387.63 ± 55.61 a | 764.79 ± 42.32 b | 297.81 ± 45.09 d | 458.48 ± 30.1 c |
| 19 | Ethyl Acetate | 893.9 | 282.756 | 1.3316 | 12,380.05 ± 281.02 a | 11,332.59 ± 110.14 b | 11,284.8 ± 557.25 b | 12,969.61 ± 252.01 a |
| 20 | Methyl acetate | 850.8 | 256.592 | 1.19848 | 516.21 ± 41.65 a | 302.11 ± 12.99 b | 125.11 ± 10.76 c | 99.69 ± 9.06 c |
| 21 | gamma-Butyrolactone | 1708.4 | 1997.64 | 1.08832 | 1430.92 ± 73.16 d | 3436.07 ± 160.6 a | 2306.96 ± 62.02 b | 1829.2 ± 45.2 c |
| 22 | Butyl pentanoate | 1299.9 | 823.328 | 1.92958 | 171.18 ± 3.44 b | 269.15 ± 20.15 a | 110.96 ± 2.68 c | 113.67 ± 4.78 c |
| 23 | Ethyl 2-methylpentanoate | 1150.1 | 572.698 | 1.76476 | 91.77 ± 2.56 b | 324.18 ± 50.62 a | 39.63 ± 1.93 b | 32.46 ± 3.24 b |
| 24 | 3-Methylbutyl propanoate | 1184.3 | 641.448 | 1.82184 | 38.84 ± 2.29 b | 142.58 ± 10.09 a | 32.21 ± 4.77 b | 28.79 ± 1.3 b |
| 25 | Ethyl heptanoate | 1358.9 | 935.774 | 1.4221 | 62.42 ± 3.17 a | 28.88 ± 2.28 b | 22.28 ± 2.36 c | 24.07 ± 1.09 bc |
| Total | 25 kinds | 57,749.18 ± 1645.53 a | 58,616.34 ± 1823.31 a | 37,526.87 ± 1142.76 c | 42,821.6 ± 487.72 b | |||
| Aldehydes | ||||||||
| 26 | Benzaldehyde | 1549.2 | 1414.329 | 1.15653 | 1222.35 ± 66.3 a | 1284.04 ± 74.14 a | 904.32 ± 108.94 b | 819.43 ± 43.81 b |
| 27 | (E)-2-Octenal | 1437.1 | 1108.822 | 1.33821 | 234.96 ± 11.23 d | 461.45 ± 21.76 b | 565.4 ± 33.75 a | 376.31 ± 19.07 c |
| 28 | Nonanal | 1400.6 | 1024.411 | 1.48034 | 257.34 ± 11.74 d | 370.74 ± 7.38 c | 528.49 ± 5.25 a | 435.71 ± 9.72 b |
| 29 | Heptanal | 1196.3 | 663.967 | 1.34493 | 490.65 ± 19.48 c | 698.53 ± 16.59 a | 633.62 ± 27.5 b | 442.62 ± 2.3 d |
| 30 | 2-Methyl-2-pentenal-M | 1174.8 | 621.574 | 1.161 | 2138.4 ± 78.52 a | 1755.37 ± 27.9 b | 672.46 ± 62.04 c | 552.39 ± 11.21 c |
| 31 | 2-Methyl-2-pentenal-D | 1174.8 | 621.574 | 1.49779 | 1612.1 ± 68.76 a | 1309.62 ± 34.02 b | 237.07 ± 14.46 c | 77.07 ± 1.34 d |
| 32 | (E)-2-Pentenal | 1144.5 | 562.112 | 1.36933 | 260.2 ± 3.76 b | 228.02 ± 3.95 c | 332.19 ± 6.15 a | 222.08 ± 10.58 c |
| 33 | (Z)-2-Pentenal-M | 1113.6 | 507.327 | 1.09329 | 2131.07 ± 53.72 a | 1937.64 ± 25.22 b | 1281.12 ± 11.41 c | 1296.05 ± 17.96 c |
| 34 | (Z)-2-Pentenal-D | 1113.6 | 507.327 | 1.35197 | 6444.13 ± 243.92 b | 7690.42 ± 89.31 a | 5870.24 ± 215.59 c | 4373.53 ± 148.63 d |
| 35 | Hexanal-M | 1097.3 | 480.602 | 1.27211 | 2102.39 ± 65.3 a | 1637.01 ± 38.43 b | 1086.82 ± 38.32 d | 1393.17 ± 14.53 c |
| 36 | Hexanal-D | 1098.1 | 481.938 | 1.56029 | 5269.69 ± 202.28 d | 7606.58 ± 168.82 b | 8467.13 ± 43.39 a | 6108.9 ± 97.85 c |
| 37 | Pentanal | 999.1 | 359.237 | 1.41739 | 2756.34 ± 98.98 c | 3283.39 ± 88.24 b | 3764.36 ± 22.79 a | 2339.38 ± 37.51 d |
| 38 | 3-Methylbutanal | 925.9 | 303.889 | 1.39964 | 5410.07 ± 242.58 a | 4268.28 ± 219.01 b | 3056.66 ± 101.49 c | 2597.13 ± 49.48 d |
| 39 | Acrolein | 862.8 | 263.636 | 1.05797 | 664.72 ± 91.54 ab | 580.02 ± 100.57 b | 910.87 ± 177.78 a | 426.15 ± 64.22 b |
| 40 | Butanal | 882.7 | 275.712 | 1.27688 | 384.91 ± 43.37 b | 331.1 ± 17.49 bc | 598.2 ± 50.91 a | 263.42 ± 8.6 c |
| 41 | Propanal | 813.6 | 235.962 | 1.12453 | 7497.44 ± 135.36 a | 7762.17 ± 12.21 a | 7030.3 ± 178.14 b | 6474.92 ± 26.1 c |
| 42 | 2-Methylpropanal | 824.8 | 242.0 | 1.27983 | 778.23 ± 68.42 a | 414.99 ± 5.78 b | 128.65 ± 32.17 c | 93.45 ± 17.79 c |
| 43 | Acetaldehyde | 763.5 | 210.804 | 0.97958 | 2479.01 ± 25.98 a | 1979.76 ± 33.2 b | 1975.78 ± 49.29 b | 1887.97 ± 75.73 b |
| 44 | 2-Methylbutanal | 910.1 | 293.323 | 1.18961 | 734.52 ± 215.47 c | 1419.16 ± 69.62 b | 2006.24 ± 105.61 a | 1884.19 ± 81.01 a |
| 45 | cis-4-Heptenal | 1245.6 | 735.435 | 1.61815 | 70.84 ± 3.19 c | 242.82 ± 15.21 c | 1029.64 ± 83.71 b | 1390.49 ± 122 a |
| 46 | 3-Methyl-2-butenal | 1213 | 687.373 | 1.09618 | 157.6 ± 25.22 ab | 133.03 ± 6.81 b | 177.42 ± 4.69 a | 135.57 ± 6.21 b |
| 47 | (E)-2-Hexenal | 1230 | 711.938 | 1.18348 | 154.8 ± 0.22 b | 170.25 ± 6.16 b | 286.31 ± 14.44 a | 153.66 ± 2.98 b |
| 48 | Diethyl acetal | 903.2 | 288.762 | 1.02714 | 276.12 ± 6.25 a | 170.46 ± 8.5 b | 126.97 ± 8.16 c | 74.28 ± 4.67 d |
| 49 | (E)-2-Heptenal | 1327.4 | 874.016 | 1.2559 | 144.33 ± 4.9 d | 232.34 ± 8.48 c | 659.91 ± 43.22 a | 414.24 ± 6.62 b |
| Total | 24 kinds | 43,672.23 ± 1224.98 b | 45,967.2 ± 562.19 a | 42,330.17 ± 255.35 b | 34,232.11 ± 266.53 c | |||
| Alcohols | ||||||||
| 50 | 1-Octanol | 1653.7 | 1774.275 | 1.48139 | 738.87 ± 20.36 a | 689.76 ± 36.41 ab | 477.77 ± 54.1 c | 600.69 ± 34.89 b |
| 51 | 1-Octen-3-ol | 1482.7 | 1224.076 | 1.16471 | 975.22 ± 48.6 a | 653.88 ± 6.77 c | 857.5 ± 10.26 b | 690.69 ± 6.14 c |
| 52 | (E)-2-Hexenol-M | 1428.2 | 1087.72 | 1.16656 | 1926.68 ± 221.16 c | 2379.84 ± 21.3 b | 3381.28 ± 95.24 a | 1952.71 ± 147.46 c |
| 53 | (E)-2-Hexenol-D | 1428.2 | 1087.72 | 1.51725 | 199.71 ± 5.13 c | 278.09 ± 14.91 b | 706 ± 53.34 a | 230.34 ± 14.03 bc |
| 54 | 1-Heptanol | 1485.1 | 1230.57 | 1.40651 | 245.94 ± 25.34 a | 213.71 ± 7.15 a | 230.92 ± 8.56 a | 254.91 ± 19.76 a |
| 55 | 1-Hexanol-M | 1367.6 | 953.629 | 1.33177 | 3793.23 ± 105.92 a | 2530.37 ± 40.78 c | 3100.05 ± 345.88 b | 3370.52 ± 95.04 ab |
| 56 | 1-Hexanol-D | 1367.6 | 953.629 | 1.64238 | 813.54 ± 51.17 a | 379.91 ± 9.37 c | 615.39 ± 140.35 b | 727.75 ± 25.9 bc |
| 57 | 3-Methyl-1-pentanol | 1337.7 | 893.617 | 1.30135 | 414.7 ± 22.04 d | 1110.91 ± 27.76 c | 2023.41 ± 78.93 a | 1795.84 ± 59.67 b |
| 58 | 1-Pentanol-M | 1263.4 | 763.001 | 1.25492 | 2197.89 ± 192.52 b | 2657.8 ± 37.39 a | 2813.86 ± 55.17 a | 2582.88 ± 82.92 a |
| 59 | 1-Pentanol-D | 1263.6 | 763.273 | 1.71603 | 902.58 ± 96.53 d | 1264.25 ± 40.14 c | 1847.54 ± 90.82 a | 1631.14 ± 68.75 b |
| 60 | 3-Methyl-1-butanol-M | 1219.2 | 696.198 | 1.24061 | 4449.66 ± 105.95 a | 4045.96 ± 41.58 b | 3601.74 ± 24.13 c | 3587.98 ± 40.99 c |
| 61 | 3-Methyl-1-butanol-D | 1219.8 | 697.069 | 1.48891 | 10,788.77 ± 254.76 b | 9589.59 ± 129.5 c | 9987.41 ± 226.35 c | 11,891.83 ± 56.3 a |
| 62 | 1-Butanol-M | 1157.8 | 587.501 | 1.18357 | 3102.04 ± 51.56 a | 2593.62 ± 6.2 b | 2463.16 ± 26.15 c | 2407 ± 19.52 c |
| 63 | 1-Butanol-D | 1158.5 | 588.837 | 1.37974 | 3233.01 ± 70.61 c | 2470.59 ± 29.61 d | 4053.32 ± 53.54 b | 4760.83 ± 13.58 a |
| 64 | 2-Methyl-1-propanol | 1104.7 | 492.628 | 1.39016 | 6306.28 ± 171.31 a | 4695.26 ± 109.49 c | 4567.56 ± 138.74 c | 5658.44 ± 24.48 b |
| 65 | 1-Propanol-M | 1050.7 | 418.611 | 1.11122 | 1882.13 ± 50.35 a | 1627.25 ± 64.08 b | 1536.6 ± 25.95 b | 1546.95 ± 36.87 b |
| 66 | 1-Propanol-D | 1051.1 | 419.114 | 1.24729 | 11,961.7 ± 478.32 a | 8549.22 ± 93.45 b | 7500.83 ± 61.2 c | 8094.03 ± 79.96 bc |
| 67 | 2-Butanol-M | 1035.8 | 400.497 | 1.14967 | 990.75 ± 49.48 b | 952.05 ± 46.01 b | 972.38 ± 28.65 b | 1165.87 ± 39.56 a |
| 68 | 2-Butanol-D | 1036.2 | 401 | 1.32569 | 640.94 ± 70.13 a | 234 ± 8.35 b | 299.43 ± 21.73 b | 285.32 ± 2.58 b |
| 69 | Ethanol | 943.2 | 315.965 | 1.13488 | 7727.46 ± 255.36 b | 7364.08 ± 131.31 b | 7454.53 ± 325.25 b | 9043.37 ± 69.68 a |
| 70 | 2-Propanol | 943.2 | 315.965 | 1.23694 | 4257.82 ± 27.13 b | 3479.58 ± 14.36 d | 4822.08 ± 144.74 a | 3735.1 ± 69.94 c |
| 71 | Furfuryl alcohol | 1733.3 | 2108.897 | 1.12724 | 1893.82 ± 134.66 a | 1881.74 ± 43.66 a | 1184.24 ± 49.39 b | 1064.05 ± 19.02 b |
| 72 | 3-Octanol | 1414.4 | 1055.589 | 1.77946 | 104.85 ± 7.25 c | 118.03 ± 5.15 c | 452.65 ± 9.74 a | 175.51 ± 1.6 b |
| Total | 23 kinds | 69,547.58 ± 1584.08 a | 59,759.48 ± 398.29 d | 64,949.65 ± 688.18 c | 67,253.74 ± 339.13 b | |||
| Ketones | ||||||||
| 73 | 1-Hydroxy-2-propanone-M | 1313.4 | 847.725 | 1.06919 | 2426.74 ± 18.5 a | 1949.15 ± 12.6 b | 1463.61 ± 52.52 c | 1238.03 ± 44.05 d |
| 74 | 1-Hydroxy-2-propanone-D | 1313.4 | 847.725 | 1.2325 | 520.19 ± 67.7 a | 394.24 ± 18.01 b | 240.12 ± 9.12 c | 220.14 ± 3.81 c |
| 75 | 3-Hydroxy-2-butanone-M | 1297.1 | 818.307 | 1.06759 | 3504.65 ± 167.54 b | 3185.82 ± 48.14 c | 3943.9 ± 10.62 a | 3767.79 ± 53.03 a |
| 76 | 3-Hydroxy-2-butanone-D | 1297.1 | 818.307 | 1.33017 | 5157.27 ± 232.7 a | 2542.07 ± 64.24 d | 4253.49 ± 284.87 b | 3684.5 ± 67.95 c |
| 77 | 2-Octanone | 1300.4 | 824.191 | 1.78488 | 1083.03 ± 76.4 a | 666.59 ± 35.04 b | 134.72 ± 4.25 c | 137.84 ± 2.82 c |
| 78 | 3-Octanone | 1263.4 | 763.001 | 1.71603 | 1235.36 ± 127.28 bc | 1059.89 ± 104.44 c | 1458.15 ± 74.36 b | 1828 ± 46.49 a |
| 79 | 2-Heptanone-M | 1191.5 | 656.984 | 1.26343 | 1357.14 ± 34.32 a | 727.85 ± 20.58 d | 1184.67 ± 28.83 b | 929.75 ± 31.56 c |
| 80 | 2-Heptanone-D | 1191.5 | 656.984 | 1.63321 | 2237.69 ± 100.71 a | 2277.34 ± 111.55 a | 1129.18 ± 39.51 b | 867.47 ± 53.61 c |
| 81 | 2,3-Pentanedione | 1065 | 436.725 | 1.25025 | 1387.07 ± 96.13 a | 958.8 ± 18.06 b | 377.64 ± 9.02 d | 561.82 ± 11.62 c |
| 82 | 2-Butanone | 911.7 | 294.329 | 1.2399 | 3117.95 ± 186.47 a | 1261.81 ± 11.48 c | 1696.4 ± 67.78 b | 1364.74 ± 10.33 c |
| 83 | Acetone | 835.7 | 248.038 | 1.11269 | 8956.62 ± 783.61 a | 5174.84 ± 149.74 b | 4568.71 ± 810.31 b | 3831.34 ± 395.24 b |
| 84 | 3-Nonanone | 1337.4 | 893.138 | 1.39632 | 197.65 ± 7.06 d | 425.63 ± 19.61 c | 506.26 ± 10.34 b | 553.58 ± 30.51 a |
| 85 | Cyclopentanone | 1147.8 | 568.305 | 1.10548 | 156.54 ± 2.94 c | 279.27 ± 13.43 a | 184.54 ± 13.71 b | 133.7 ± 9.83 c |
| Total | 13 kinds | 31,337.91 ± 1695.68 a | 20,903.29 ± 81.83 b | 21,141.37 ± 683.66 b | 19,118.7 ± 406.56 b | |||
| Heterocyclic compounds | ||||||||
| 86 | 3-Ethylpyridine | 1386.5 | 993.568 | 1.10565 | 494.71 ± 9.31 a | 508.7 ± 18.96 a | 206.13 ± 23.53 b | 212.88 ± 1.73 b |
| 87 | 2,3-Dimethylpyrazine | 1349 | 915.974 | 1.11082 | 555.23 ± 28.29 a | 412.82 ± 14.69 b | 196.94 ± 3.82 c | 177.47 ± 4.54 c |
| 88 | 2,5-Dimethylpyrazine | 1325.4 | 870.082 | 1.12042 | 438.9 ± 4.51 a | 172.45 ± 7 b | 63.12 ± 7.48 c | 57.8 ± 2.26 c |
| 89 | 2-Methylpyrazine | 1275.2 | 781.829 | 1.09641 | 453.58 ± 23.77 a | 214.37 ± 2.85 b | 147.87 ± 7.17 c | 134.66 ± 4.4 c |
| 90 | 2-Pentylfuran | 1241 | 728.429 | 1.2509 | 1482.58 ± 54.72 a | 1049.54 ± 24.67 b | 743.91 ± 30.97 c | 773.04 ± 6.68 c |
| 91 | 2-Butylfuran | 1107.6 | 497.305 | 1.17142 | 876.46 ± 5.58 a | 706.88 ± 19.12 b | 509.37 ± 7.29 d | 657.32 ± 16.49 c |
| 92 | 2,5-Dimethylfuran | 946.7 | 318.481 | 1.37745 | 2174.36 ± 141.44 a | 1152.76 ± 62.46 b | 1978.58 ± 307.44 a | 317.52 ± 25.71 c |
| 93 | 2-Ethylfuran | 958.4 | 327.035 | 1.28871 | 252.83 ± 22.01 a | 74.43 ± 5.81 c | 117.04 ± 3.49 b | 61.61 ± 2.85 c |
| 94 | Pyridine | 1170.9 | 613.68 | 1.2635 | 112.19 ± 18.39 d | 150.43 ± 4.62 c | 616.37 ± 2.79 a | 216.79 ± 1.95 b |
| 95 | Thiophene | 1027 | 390.207 | 1.0475 | 1613.63 ± 39.07 a | 1001.38 ± 17.84 d | 1118.64 ± 79.38 c | 1378.14 ± 29.08 b |
| Total | 10 kinds | 8454.45 ± 313.69 a | 5443.76 ± 82.71 b | 5697.97 ± 228.32 b | 3987.23 ± 37.23 c | |||
| Carboxylic acids | ||||||||
| 96 | Butanoic acid-M | 1712.9 | 2017.481 | 1.16247 | 37,635.9 ± 732.85 a | 20,863.3 ± 2142.89 b | 18,271.38 ± 519.5 b | 18,186.25 ± 147.07 b |
| 97 | Butanoic acid-D | 1712.4 | 2015.05 | 1.36848 | 28,868.24 ± 1837.27 a | 52,63.79 ± 1187.17 b | 4516.87 ± 242.04 b | 4668.38 ± 72.59 b |
| 98 | 2-Methylpropanoic acid-M | 1640.3 | 1723.202 | 1.15059 | 23,690.86 ± 384.85 a | 11,082.69 ± 884.89 c | 21,625.23 ± 915.42 b | 23,702.71 ± 281.66 a |
| 99 | 2-Methylpropanoic acid-D | 1640.9 | 1725.634 | 1.37245 | 7659.53 ± 811.85 a | 1517.3 ± 250.71 c | 5605.63 ± 474.18 b | 6759.93 ± 141.04 ab |
| 100 | Propanoic acid-M | 1633.7 | 1698.881 | 1.11889 | 3317.51 ± 51.9 b | 4065.17 ± 57.08 a | 1145.18 ± 20.61 c | 967.87 ± 39.12 d |
| 101 | Propanoic acid-D | 1635 | 1703.745 | 1.29519 | 1833 ± 104.99 a | 1075.68 ± 95.15 b | 749.73 ± 14 c | 623.17 ± 114.39 c |
| 102 | Acetic acid-M | 1500.8 | 1273.27 | 1.05947 | 30,454.85 ± 730.64 a | 29,370.65 ± 488.98 a | 28,918.44 ± 1048 a | 29,322.27 ± 185.48 a |
| 103 | Acetic acid-D | 1500.8 | 1273.27 | 1.15653 | 36,224.97 ± 190.56 b | 39,720.15 ± 185.77 a | 26,958.14 ± 367.99 c | 23,843.66 ± 830.55 d |
| Total | 8 kinds | 169,684.85 ± 3386.03 a | 112,958.72 ± 5135.23 b | 107,790.6 ± 3056.05 b | 108,074.25 ± 964.34 b | |||
| Terpenes | ||||||||
| 104 | gamma-Terpinene | 1246.7 | 737.14 | 1.22592 | 471.25 ± 14.58 d | 785.15 ± 22.78 c | 1505.13 ± 48.29 b | 1666.59 ± 35.72 a |
| 105 | alpha-Terpinene | 1189.3 | 652.308 | 1.22697 | 1356.99 ± 15.96 b | 1484.76 ± 28.85 a | 831.85 ± 41.68 d | 1031.31 ± 57.85 c |
| 106 | Myrcene-M | 1180.6 | 633.6 | 1.22697 | 1075.64 ± 21.45 a | 541.83 ± 5.87 b | 115.37 ± 8.11 c | 90.54 ± 1.19 c |
| 107 | Myrcene-D | 1180.6 | 633.6 | 1.64015 | 326.92 ± 9.47 a | 144.96 ± 7.73 b | 65.26 ± 1.36 c | 44.37 ± 0.7 d |
| 108 | beta-Pinene | 1144.8 | 562.78 | 1.21655 | 623.32 ± 14.21 a | 542.98 ± 22.48 b | 382.47 ± 7.39 d | 422.19 ± 5.49 c |
| 109 | alpha-Pinene | 1026.8 | 389.93 | 1.28723 | 610.47 ± 41.58 a | 322.49 ± 5.34 b | 301.2 ± 5.51 b | 287.75 ± 3.08 b |
| 110 | p-Cymene | 1302.5 | 827.905 | 1.29549 | 388.01 ± 16.68 a | 427.03 ± 30.2 a | 161.29 ± 6.63 b | 138.19 ± 4.61 b |
| Total | 7 kinds | 4852.6 ± 122.64 a | 4249.2 ± 65.77 b | 3362.57 ± 70.83 d | 3680.95 ± 89.49 c | |||
| Sulfur-containing compounds | ||||||||
| 111 | Dimethyl trisulfide | 1412.9 | 1052.007 | 1.3013 | 183.99 ± 9.1 d | 396.61 ± 6.65 c | 1385.08 ± 25.62 a | 590.74 ± 26.53 b |
| 112 | Dimethyl disulfide | 1048.8 | 416.246 | 1.15241 | 503.64 ± 100.66 b | 1064.68 ± 24.48 a | 935.1 ± 49.2 a | 1035.39 ± 35.01 a |
| 113 | Methyl 2-propenyl sulfide | 982.4 | 345.181 | 1.03654 | 639.33 ± 20.79 b | 383.92 ± 11.65 d | 706.6 ± 15.61 a | 474.33 ± 7.24 c |
| Total | 3 kinds | 1326.96 ± 88.41 d | 1845.21 ± 15.2 c | 3026.78 ± 64.68 a | 2100.46 ± 19.72 b | |||
| Nitrogen-containing compounds | ||||||||
| 114 | Trimethylamine | 837.5 | 249.044 | 1.14524 | 13,163.86 ± 1177.72 c | 20,155.58 ± 621.09 b | 25,675.48 ± 2361.68 a | 27,593.84 ± 1417.5 a |
| 115 | Ammonia | 1260.2 | 757.916 | 0.85109 | 3525.72 ± 635.6 a | 2840.95 ± 260.16 ab | 2313.13 ± 105.74 b | 2771.16 ± 205.98 ab |
| Total | 2 kinds | 16,689.58 ± 1745.14 c | 22,996.53 ± 371.18 b | 27,988.61 ± 2256.53 a | 30,365 ± 1623.45 a | |||
| Aromatic hydrocarbons | ||||||||
| 116 | p-Xylene | 1144.5 | 562.112 | 1.07593 | 760.66 ± 23.35 b | 719.13 ± 15.16 b | 850.45 ± 52.77 a | 419.44 ± 14.21 c |
| Total | 1 kind | 760.66 ± 23.35 b | 719.13 ± 15.16 b | 850.45 ± 52.77 a | 419.44 ± 14.21 c | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, P.; Li, L.; Wei, Y.; Sun, J.; Tang, B.; Yang, Y.; Chen, J.; Wu, L. GC-IMS-Based Volatile Characteristic Analysis of Hypsizygus marmoreus Dried by Different Methods. Foods 2024, 13, 1322. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13091322
Lai P, Li L, Wei Y, Sun J, Tang B, Yang Y, Chen J, Wu L. GC-IMS-Based Volatile Characteristic Analysis of Hypsizygus marmoreus Dried by Different Methods. Foods. 2024; 13(9):1322. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13091322
Chicago/Turabian StyleLai, Pufu, Longxiang Li, Yingying Wei, Junzheng Sun, Baosha Tang, Yanrong Yang, Junchen Chen, and Li Wu. 2024. "GC-IMS-Based Volatile Characteristic Analysis of Hypsizygus marmoreus Dried by Different Methods" Foods 13, no. 9: 1322. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13091322