Electronic Tongue as a Correlative Technique for Modeling Cattle Meat Quality and Classification of Breeds
Abstract
:1. Introduction
2. Materials and Methods
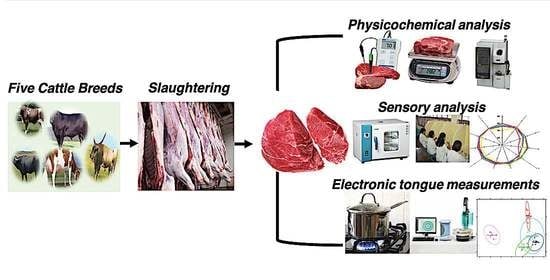
2.1. Sampling and Experimental Design
2.2. Physico-Chemical Analysis
2.2.1. Determination of pH
2.2.2. Determination of Color
2.2.3. Determination of Water-Holding Capacity (WHC)
2.2.4. Determination of Water Activity
2.2.5. Determination of Dry Matter-Content
2.2.6. Texture Analyses
2.3. Sensory Evaluation
2.4. E-Tongue Analysis
2.5. Statistical Data Analysis
3. Results
3.1. Physico-Chemical Analysis
3.1.1. pH
3.1.2. Color
3.1.3. Water-Holding Capacity
3.1.4. Water Activity
3.1.5. Dry Matter Content
3.1.6. Warner–Bratzler Shear Force
3.2. Sensory Evaluation
3.3. Classification of Meat Breeds with E-Tongue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonnet, C.; Bouamra-Mechemache, Z.; Réquillart, V.; Treich, N. Viewpoint: Regulating meat consumption to improve health, the environment and animal welfare. Food Policy 2020, 97, 101847. [Google Scholar] [CrossRef]
- European Union Regulation (EU). No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council. Off. J. Eur. Union 2011, 304, 18–62. [Google Scholar]
- Hozáková, K.; Vavrišínová, K.; Neirurerová, P.; Bujko, J. Growth of beef cattle as prediction for meat production: A review. Acta Fytotech. Zootech. 2020, 23, 58–69. [Google Scholar] [CrossRef]
- Holló, G.; Nuernberg, K.; Somogyi, T.; Anton, I.; Holló, I. Comparison of fattening performance and slaughter value of local Hungarian cattle breeds to international breeds. Arch. Anim. Breed. 2012, 55, 1–12. [Google Scholar] [CrossRef]
- Prommachart, R.; Belem, T.S.; Uriyapongson, S.; Rayas-Duarte, P.; Uriyapongson, J.; Ramanathan, R. The effect of black rice water extract on surface color, lipid oxidation, microbial growth, and antioxidant activity of beef patties during chilled storage. Meat Sci. 2020, 164, 108091. [Google Scholar] [CrossRef]
- Tomasevic, I.; Djekic, I.; Font-i-Furnols, M.; Terjung, N.; Lorenzo, J.M. Recent advances in meat color research. Curr. Opin. Food Sci. 2021, 41, 81–87. [Google Scholar] [CrossRef]
- Chambaz, A.; Scheeder, M.R.L.; Kreuzer, M.; Dufey, P.A. Meat quality of Angus, Simmental, Charolais and Limousin steers compared at the same intramuscular fat content. Meat Sci. 2003, 63, 491–500. [Google Scholar] [CrossRef]
- Pesonen, M.; Honkavaara, M.; Huuskonen, A. Effect of breed on production, carcass traits and meat quality of aberdeen angus, limousin and aberdeen angus×limousin bulls offered a grass silage-grain-based diet. Agric. Food Sci. 2012, 21, 361–369. [Google Scholar] [CrossRef]
- Bureš, D.; Bartoň, L. Muscle sampling and analyses. Czech J. Anim. Sci. 2012, 57, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Szmańko, T.; Lesiów, T.; Górecka, J. The water-holding capacity of meat: A reference analytical method. Food Chem. 2021, 357, 129727. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Vitalis, F.; Zaukuu, J.L.Z.; Bodor, Z.; Aouadi, B.; Hitka, G.; Kaszab, T.; Zsom-Muha, V.; Gillay, Z.; Kovacs, Z. Detection and quantification of tomato paste adulteration using conventional and rapid analytical methods. Sensors 2020, 20, 6059. [Google Scholar] [CrossRef] [PubMed]
- Bodor, Z.; Koncz, F.A.; Zaukuu, J.-L.Z.; Kertész, I.; Gillay, Z.; Kaszab, T.; Kovacs, Z.; Benedek, C. Effect of heat treatment on chemical and sensory properties of honeys. Anim. Welf. Ethol. Hous. Syst. 2018, 13, 39–48. [Google Scholar] [CrossRef]
- Zaukuu, J.L.Z.; Bodor, Z.; Vitalis, F.; Zsom-Muha, V.; Kovacs, Z. Near infrared spectroscopy as a rapid method for detecting paprika powder adulteration with corn flour. Acta Period. Technol. 2019, 50, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Marx, Í.M.G.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Drunkler, D.A.; Peres, A.M. Quantification of table olives’ acid, bitter and salty tastes using potentiometric electronic tongue fingerprints. LWT—Food Sci. Technol. 2017, 79, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, X.; Liu, Y.; Xu, X.; Zhou, G. Isolation and identification of flavour peptides from Puffer fish (Takifugu obscurus) muscle using an electronic tongue and MALDI-TOF/TOF MS/MS. Food Chem. 2012, 135, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- González-Calabuig, A.; del Valle, M. Voltammetric electronic tongue to identify Brett character in wines. On-site quantification of its ethylphenol metabolites. Talanta 2018, 179, 70–74. [Google Scholar] [CrossRef]
- Magdalena, S.; Wardencki, W. Food Analysis Using Artificial Senses. J. Agric. Food Chem. 2015, 6, 80–233. [Google Scholar]
- Kovacs, Z.; Szöllősi, D.; Zaukuu, J.-L.Z.; Bodor, Z.; Vitális, F.; Aouadi, B.; Zsom-Muha, V.; Gillay, Z. Factors Influencing the Long-Term Stability of Electronic Tongue and Application of Improved Drift. Biosensors 2020, 10, 74. [Google Scholar] [CrossRef]
- Zaukuu, J.L.Z.; Gillay, Z.; Kovacs, Z. Standardized extraction techniques for meat analysis with the electronic tongue: A case study of poultry and red meat adulteration. Sensors 2021, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Zaukuu, J.-L.Z.; Bazar, G.; Gillay, Z.; Kovacs, Z. Emerging trends of advanced sensor based instruments for meat, poultry and fish quality—A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 3443–3460. [Google Scholar] [CrossRef] [PubMed]
- Haddi, Z.; El Barbri, N.; Tahri, K.; Bougrini, M.; El Bari, N.; Llobet, E.; Bouchikhi, B.; El Barbri, N.; Tahri, K.; Bougrini, M.; et al. Instrumental assessment of red meat origins and their storage time using electronic sensing systems. Anal. Methods 2015, 7, 5193–5203. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y.; Meng, Q.; Li, N.; Ren, L. Evaluation of Beef by Electronic Tongue System TS-5000Z: Flavor Assessment, Recognition and Chemical Compositions According to Its Correlation with Flavor. PLoS ONE 2015, 10, e0137807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Xu, B.; Yu, H. Electronic nose and electronic tongue combined with fuzzy mathematics sensory evaluation to optimize bacon smoking procedure. Sci. Agric. Sin. 2017, 50, 3864. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, S.; Wang, N.; Deng, Y.; Sha, L.; Gai, S.; Liu, H.; Xu, X. Evolution of Taste Compounds of Dezhou-Braised Chicken During Cooking Evaluated by Chemical Analysis and an Electronic Tongue System. J. Food Sci. 2017, 82, 1076–1082. [Google Scholar] [CrossRef]
- Aroeira, C.N.; de Almeida Torres Filho, R.; Fontes, P.R.; de Lemos Souza Ramos, A.; de Miranda Gomide, L.A.; Ladeira, M.M.; Ramos, E.M. Effect of freezing prior to aging on myoglobin redox forms and CIE color of beef from Nellore and Aberdeen Angus cattle. Meat Sci. 2017, 125, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1995; ISBN 1546-4121. [Google Scholar]
- Dos Santos, P.V.; Paris, W.; de Menezes, L.F.G.; Vonz, D.; da Silveira, M.F.; Tubin, J. Carcass physical composition and meat quality of holstein calves, terminated in different finishing systems and slaughter weights. Ciênc. Agrotecnol. 2013, 37, 443–450. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization (ISO). ISO 13299; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- International Organization for Standardization (ISO). ISO 4121; ISO: Geneva, Switzerland, 1987. [Google Scholar]
- Kókai, Z.; Heszberger, J.; Kollár-Hunek, K.; Szabó, R.; Papp, E. ProfiSens—A profile analysis supporting software in food industry, related research and education. Period. Polytech. Chem. Eng. 2004, 48, 31–41. [Google Scholar]
- Hwang, I.H.; Thompson, J.M. Effects of pH early postmortem on meat quality in beef longissimus. Asian-Australas. J. Anim. Sci. 2003, 16, 1218–1223. [Google Scholar] [CrossRef]
- McWilliams, M. Meats, Poultry, and Fish. In Foods: Experimental Perspectives; California State University: Los Angeles, CA, USA, 2012; pp. 253–285. ISBN 9780137079292. [Google Scholar]
- Bartosiewicz, L. The Hungarian Grey cattle: A traditional European breed. Anim. Genet. Resour. Inf. 1997, 21, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Warner, R. Measurements of Water-holding Capacity and Color: Objective and Subjective. In Encyclopedia of Meat Sciences; Academic Press: Waltham, MA, USA, 2014; pp. 164–171. [Google Scholar] [CrossRef]
- Barker, J.S.F. Encyclopedia of Global Archaeology. In Encyclopedia of Global Archaeology; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Li, P.; Wang, T.; Mao, Y.; Zhang, Y.; Niu, L.; Liang, R.; Zhu, L.; Luo, X. Effect of Ultimate pH on Postmortem Myofibrillar Protein Degradation and Meat Quality Characteristics of Chinese Yellow Crossbreed Cattle. Sci. World J. 2014, 2014, 174253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveena, B.M.; Kiran, M. Buffalo meat quality, composition, and processing characteristics: Contribution to the global economy and nutritional security. Anim. Front. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Blott, S.C.; Williams, J.L.; Haley, C.S. Discriminating among cattle breeds using genetic markers. Heredity 1999, 82, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Bressan, M.C.; Rodrigues, E.C.; Rossato, L.V.; Neto-Fonseca, I.; Alves, S.P.; Bessa, R.J.B.; Gama, L.T. Discrimination of meat produced by bos taurus and bos indicus finished under an intensive or extensive system. Animals 2020, 10, 1737. [Google Scholar] [CrossRef]
- Hrbek, V.; Zdenkova, K.; Jilkova, D.; Cermakova, E.; Jiru, M.; Demnerova, K.; Pulkrabova, J.; Hajslova, J. Authentication of meat and meat products using triacylglycerols profiling and by DNA analysis. Foods 2020, 9, 1269. [Google Scholar] [CrossRef] [PubMed]
- Berri, C.; Picard, B.; Lebret, B.; Andueza, D.; Lefèvre, F.; Le Bihan-Duval, E.; Beauclercq, S.; Chartrin, P.; Vautier, A.; Legrand, I.; et al. Predicting the quality of meat: Myth or reality? Foods 2019, 8, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Angus | Holstein | Domestic Buffalo | Hungarian Grey | Hungarian Spotted | ||
|---|---|---|---|---|---|---|
| Average recognition: 100% | Angus | 100 | 0 | 0 | 0 | 0 |
| Holstein | 0 | 100 | 0 | 0 | 0 | |
| Domestic buffalo | 0 | 0 | 100 | 0 | 0 | |
| Hungarian Grey | 0 | 0 | 0 | 100 | 0 | |
| Hungarian Spotted | 0 | 0 | 0 | 0 | 100 | |
| Angus | Holstein | Domestic Buffalo | Hungarian Grey | Hungarian Spotted | ||
| Average prediction: 97.52% | Angus | 100 | 0 | 0 | 0 | 0 |
| Holstein | 0 | 100 | 0 | 0 | 0 | |
| Domestic buffalo | 0 | 0 | 87.59 | 0 | 0 | |
| Hungarian Grey | 0 | 0 | 0 | 100 | 0 | |
| Hungarian Spotted | 0 | 0 | 12.41 | 0 | 100 |
| Parameter | R2C | RMSEC | R2CV | RMSECV |
|---|---|---|---|---|
| pH | 0.89 | 0.07 | 0.73 | 0.10 |
| Water activity | 0.78 | 0.01 | 0.58 | 0.01 |
| Dry matter content [%(m/m)] | 0.78 | 0.01 | 0.58 | 0.01 |
| Water-holding capacity [mm2/mg] | ** | ** | ** | ** |
| Color a* | ** | ** | ** | ** |
| Color b* | ** | ** | ** | ** |
| Color L* | ** | ** | ** | ** |
| Force raw [g] | ** | ** | ** | ** |
| Force roasted [g] | ** | ** | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surányi, J.; Zaukuu, J.-L.Z.; Friedrich, L.; Kovacs, Z.; Horváth, F.; Németh, C.; Kókai, Z. Electronic Tongue as a Correlative Technique for Modeling Cattle Meat Quality and Classification of Breeds. Foods 2021, 10, 2283. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102283
Surányi J, Zaukuu J-LZ, Friedrich L, Kovacs Z, Horváth F, Németh C, Kókai Z. Electronic Tongue as a Correlative Technique for Modeling Cattle Meat Quality and Classification of Breeds. Foods. 2021; 10(10):2283. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102283
Chicago/Turabian StyleSurányi, József, John-Lewis Zinia Zaukuu, László Friedrich, Zoltan Kovacs, Ferenc Horváth, Csaba Németh, and Zoltán Kókai. 2021. "Electronic Tongue as a Correlative Technique for Modeling Cattle Meat Quality and Classification of Breeds" Foods 10, no. 10: 2283. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102283