Comprehensive Assessment of Thermochemical Processes for Sustainable Waste Management and Resource Recovery
Abstract
:1. Introduction
1.1. Hydrothermal Liquefaction: Parameters and Applications
- Parameters:
- Purposes of Hydrothermal Liquefaction:
1.2. Hydrothermal Carbonisation: Parameters and Applications
- Parameters:
- Purposes and Applications:
1.3. Pyrolysis: Parameters and Applications
- Parameters:
- Purposes and Applications:
1.4. Gasification: Parameters and Applications
- Parameters:
- Purposes and Applications:
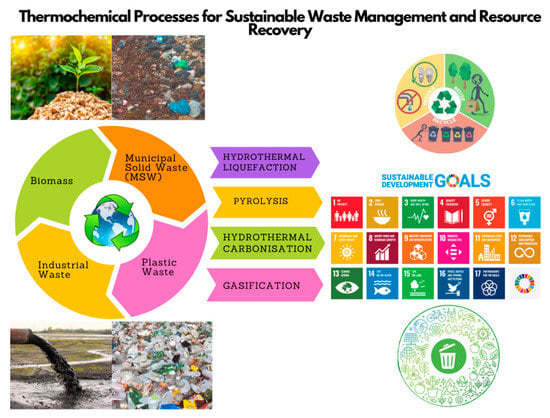
2. Waste Materials and General Classification
2.1. Biomass
2.2. Municipal Solid Waste (MSW)
2.3. Plastic Waste
2.4. Industrial Waste
3. Waste Production Problem and Sustainability
3.1. Biomass
- Hydrothermal Liquefaction (HTL): Different feedstocks and reaction parameters are used for HTL process. The higher heating values (HHVs) vary depending on different catalysts and reaction conditions used. HTL process enables the production of hydrocarbons with high HHVs for various feedstocks. Particularly, feedstocks such as ammi visnaga and birchwood sawdust may be suitable for obtaining high HHVs. In summary:
- It offers suitable HHVs for various feedstocks;
- The availability of feedstocks can vary widely;
- Catalyst costs and process conditions should be economically evaluated.
- Hydrothermal Carbonization (HTC): The HTC process is used for the carbonization of various biomasses. HHVs of the hydrocarbon obtained through HTC process vary for different biomasses. The HTC process provides medium HHVs, especially for feedstocks like coconut fiber and dead eucalyptus leaves. This method allows carbonization under low temperature and pressure conditions.
- It provides medium HHVs;
- It can be suitable for various biomass sources;
- Energy costs are generally low due to low process temperatures and pressures.
- Pyrolysis: The pyrolysis process is carried out using different feedstocks and temperature ranges. The use of different catalysts and reaction conditions affects the HHVs of pyrolysis products. Pyrolysis enables the production of products with high HHVs from feedstocks like pruning residues. Pyrolysis, conducted at high temperatures, results in the thermal decomposition of biomass into gas, liquid, and solid products.
- It offers high HHVs and yields energy-intensive products;
- Biomass sources generally have widespread accessibility;
- The high temperature requirement of the pyrolysis process can increase energy costs.
- Gasification: The gasification process is performed using various feedstocks and temperature ranges. The catalysts and gasification conditions used affect the HHVs of gasification products. Gasification process provides gas production with varying HHVs for different feedstocks. Especially, feedstocks like sawdust and pelletized sawdust can yield high-energy gas through gasification.
- It offers varying HHVs for different gasification sources;
- It has a wide range of biomass sources;
- High temperature and pressure requirements can affect energy costs.
- Economic factors:
- The cost and availability of feedstocks affect the conversion processes;
- Locally available and low-cost feedstocks may be economically preferred.
- Availability factors:
- The availability of biomass sources can vary regionally and seasonally;
- Available resources in the region where the conversion processes will be used should be taken into account.
3.2. Municipal Solid Waste (MSW)
- Hydrothermal Liquefaction (HTL):
- Feedstocks, such as biopulp, biogenic waste, food waste, fruit and agricultural wastes, and sewage sludge, can be effectively converted using HTL;
- Catalysts, such as K2CO3, Na2CO3, and CeZrOx, are used in the HTL process;
- HHVs range from 18.86 MJ/kg to 38.19 MJ/kg for the mentioned feedstocks.
- Hydrothermal Carbonization (HTC):
- Municipal mixed waste, mixed municipal solid waste, sewage sludge, municipal solid waste pulp, and chicken manure are suitable feedstocks for HTC;
- HTC is typically conducted at temperatures ranging from 140 °C to 300 °C;
- The HHVs for the mentioned feedstocks range from 10.11 MJ/kg to 21.5 MJ/kg.
- Pyrolysis:
- MSW is a mixture of plastic, paper, textile, and other organic wastes; biomass; plastic; paper; cardboard; and the organic fraction of MSW that can be converted through pyrolysis;
- Pyrolysis temperatures typically range from 350 °C to 600 °C;
- The HHVs for the mentioned feedstocks range from 17.77 MJ/kg to 50.69 MJ/kg.
- Gasification:
- Kitchen garbage, paper, cloth and fiber, plastics, and other organic wastes can be subjected to gasification;
- Gasification temperatures can range from 200 °C to 1200 °C;
- The availability and economy of raw materials should be considered when choosing the appropriate gasification method.
3.3. Plastic Waste
- Hydrothermal Liquefaction (HTL):
- Various types of plastics, such as PET, PC, polyamide 6 (PA6), polyurethane (PU), PE, PP, and PS, can be converted using HTL;
- Reaction temperatures for HTL range from 100 °C to 450 °C;
- Catalysts, such as NaOH, KOH, Na2CO3, and diaminotoluene (TDA), are used in the HTL process;
- The HHVs for the mentioned plastics range from 17.43 MJ/kg to 49 MJ/kg.
- Hydrothermal Carbonization (HTC):
- Plastic waste, PVC waste, PVC, and various plastic mixtures can be subjected to HTC;
- HTC is typically conducted at temperatures ranging from 180 °C to 300 °C;
- The availability and economy of raw materials should be considered when choosing the appropriate HTC method.
- Pyrolysis:
- Plastic waste, such as HDPE, LDPE, IPP, PS, ABS, PP, and PVC can be effectively converted through pyrolysis;
- Pyrolysis temperatures typically range from 290 °C to 730 °C;
- Catalysts used in pyrolysis include HZSM-5, PZSM-5, Ziegler-Natta (Z-N), Ni/h-ZSM-5, and activated carbon;
- The HHVs for the mentioned plastics range from 36.3 MJ/kg to 44.2 MJ/kg.
- Gasification:
- PE, PP, and other plastic mixtures can undergo gasification;
- Gasification temperatures can range from 500 °C to 900 °C;
- Various catalysts, such as NiO/ɤ-Al2O3, dolomite, olivine, and Ca(OH)2 can be used in the gasification process;
- The availability and economy of raw materials should be considered when choosing the appropriate gasification method.
3.4. Industrial Waste
- Hydrothermal Liquefaction (HTL):
- Various types of domestic solid waste, such as food waste, tobacco-processing waste, oil palm fruit press fiber, shrimp shells, and olive oil waste, can be converted using HTL;
- Reaction temperatures for HTL range from 90 °C to 350 °C;
- The availability and economy of raw materials should be considered when choosing the appropriate HTL method.
- Hydrothermal Carbonization (HTC):
- Domestic solid waste, such as food waste, crude oil sludge, oily sludge, olive mill wastewater, and various agro-industrial wastes, can be subjected to HTC;
- HTC is typically conducted at temperatures ranging from 150 °C to 275 °C;
- The availability and economy of raw materials should be considered when choosing the appropriate HTC method.
- Pyrolysis:
- Domestic solid waste, such as olive oil industry waste, industrial wastes, tire industry wastes, and various agricultural and industrial wastes, can be effectively converted through pyrolysis;
- Pyrolysis temperatures typically range from 300 °C to 700 °C;
- The availability and economy of raw materials should be considered when choosing the appropriate pyrolysis method.
- Gasification:
- Domestic solid waste, such as palm kernel shell–oil sludge mixtures, bagasse residue, olive pomace, oily sludge, waste tires, and various industrial and municipal sludges, can undergo gasification;
- Gasification temperatures can range from 400 °C to 1000 °C;
- The availability and economy of raw materials should be considered when choosing the appropriate gasification method.
- Economic Factors:
- Availability Factors:
4. General Situation and Forecasts in the World
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seo, M.W.; Lee, S.H.; Nam, H.; Lee, D.; Tokmurzin, D.; Wang, S.; Park, Y.-K. Recent Advances of Thermochemical Conversion Processes for Biorefinery. Bioresour. Technol. 2022, 343, 126109. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Abu-Jdayil, B. Crude Glycerol as a Potential Feedstock for Future Energy via Thermochemical Conversion Processes: A Review. Sustainability 2021, 13, 12813. [Google Scholar] [CrossRef]
- Tews, I.J.; Garcia-Perez, M. Advanced Oxidative Techniques for the Treatment of Aqueous Liquid Effluents from Biomass Thermochemical Conversion Processes: A Review. Energy Fuels 2022, 36, 60–79. [Google Scholar] [CrossRef]
- Genel, S.; Durak, H.; Durak, E.D.; Güneş, H.; Genel, Y. Hydrothermal Liquefaction of Biomass with Molybdenum, Aluminum, Cobalt Metal Powder Catalysts and Evaluation of Wastewater by Fungus Cultivation. Renew Energy 2023, 203, 20–32. [Google Scholar] [CrossRef]
- Mathanker, A.; Das, S.; Pudasainee, D.; Khan, M.; Kumar, A.; Gupta, R. A Review of Hydrothermal Liquefaction of Biomass for Biofuels Production with a Special Focus on the Effect of Process Parameters, Co-Solvents, and Extraction Solvents. Energies 2021, 14, 4916. [Google Scholar] [CrossRef]
- Venkatachalam, C.D.; Ravichandran, S.R.; Sengottian, M. Lignocellulosic and Algal Biomass for Bio-Crude Production Using Hydrothermal Liquefaction: Conversion Techniques, Mechanism and Process Conditions: A Review. Environ. Eng. Res. 2021, 27, 200555. [Google Scholar] [CrossRef]
- Shen, Y. A Review on Hydrothermal Carbonization of Biomass and Plastic Wastes to Energy Products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Bardhan, M.; Novera, T.M.; Tabassum, M.; Islam, M.A.; Islam, M.A.; Hameed, B.H. Co-Hydrothermal Carbonization of Different Feedstocks to Hydrochar as Potential Energy for the Future World: A Review. J. Clean Prod. 2021, 298, 126734. [Google Scholar] [CrossRef]
- Li, Q.; Faramarzi, A.; Zhang, S.; Wang, Y.; Hu, X.; Gholizadeh, M. Progress in Catalytic Pyrolysis of Municipal Solid Waste. Energy Convers. Manag. 2020, 226, 113525. [Google Scholar] [CrossRef]
- Du, Y.; Ju, T.; Meng, Y.; Lan, T.; Han, S.; Jiang, J. A Review on Municipal Solid Waste Pyrolysis of Different Composition for Gas Production. Fuel Process. Technol. 2021, 224, 107026. [Google Scholar] [CrossRef]
- Zaccariello, L.; Montagnaro, F. Fluidised Bed Gasification of Biomasses and Wastes to Produce Hydrogen-Rich Syn-Gas—A Review. J. Chem. Technol. Biotechnol. 2023, 98, 1878–1887. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.U.; Gunes, B. Waste Biomass Valorization for the Production of Biofuels and Value-Added Products: A Comprehensive Review of Thermochemical, Biological and Integrated Processes. Process Saf. Environ. Prot. 2022, 159, 323–344. [Google Scholar] [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Sánchez, J.; Curt, M.D.; Robert, N.; Fernández, J. Chapter Two—Biomass Resources. In The Role of Bioenergy in the Bioeconomy; Lago, C., Caldés, N., Lechón, Y., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 25–111. ISBN 978-0-12-813056-8. [Google Scholar]
- Lim, C.H.; Lam, H.L. Biomass Supply Chain Optimisation via Novel Biomass Element Life Cycle Analysis (BELCA). Appl. Energy 2016, 161, 733–745. [Google Scholar] [CrossRef]
- Pan, H. Synthesis of Polymers from Organic Solvent Liquefied Biomass: A Review. Renew. Sustain. Energy Rev. 2011, 15, 3454–3463. [Google Scholar] [CrossRef]
- Lu, J.-S.; Chang, Y.; Poon, C.-S.; Lee, D.-J. Slow Pyrolysis of Municipal Solid Waste (MSW): A Review. Bioresour. Technol. 2020, 312, 123615. [Google Scholar] [CrossRef]
- Ashani, P.N.; Shafiei, M.; Karimi, K. Biobutanol Production from Municipal Solid Waste: Technical and Economic Analysis. Bioresour. Technol. 2020, 308, 123267. [Google Scholar] [CrossRef] [PubMed]
- Arena, U. Process and Technological Aspects of Municipal Solid Waste Gasification. A Review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A Technical Review of Bioenergy and Resource Recovery from Municipal Solid Waste. J. Hazard Mater. 2021, 403, 123970. [Google Scholar] [CrossRef]
- Lomwongsopon, P.; Varrone, C. Critical Review on the Progress of Plastic Bioupcycling Technology as a Potential Solution for Sustainable Plastic Waste Management. Polymers 2022, 14, 4996. [Google Scholar] [CrossRef]
- Alam, S.S.; Husain Khan, A.; Khan, N.A. Plastic Waste Management via Thermochemical Conversion of Plastics into Fuel: A Review. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 1–20. [Google Scholar] [CrossRef]
- Prajapati, R.; Kohli, K.; Maity, S.K.; Sharma, B.K. Potential Chemicals from Plastic Wastes. Molecules 2021, 26, 3175. [Google Scholar] [CrossRef]
- Yansaneh, O.Y.; Zein, S.H. Latest Advances in Waste Plastic Pyrolytic Catalysis. Processes 2022, 10, 683. [Google Scholar] [CrossRef]
- Viganó, J.; Machado, A.P.d.F.; Martínez, J. Sub- and Supercritical Fluid Technology Applied to Food Waste Processing. J. Supercrit. Fluids 2015, 96, 272–286. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Loureiro, L.M.E.F.; Sá, L.C.R.; Silva, H.F.C. Thermochemical Conversion of Olive Oil Industry Waste: Circular Economy through Energy Recovery. Recycling 2020, 5, 12. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Loureiro, L.M.E.F.; Sá, L.C.R.; Silva, H.F.C. Sugarcane Industry Waste Recovery: A Case Study Using Thermochemical Conversion Technologies to Increase Sustainability. Appl. Sci. 2020, 10, 6481. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ruiz, B.; Martínez-Blanco, D.; Sánchez-Arenillas, M.; Diez, M.A.; Suárez-Ruiz, I.; Marco, J.F.; Blanco, J.; Fuente, E. Sustainable Thermochemical Single-Step Process to Obtain Magnetic Activated Carbons from Chestnut Industrial Wastes. ACS Sustain. Chem. Eng. 2019, 7, 17293–17305. [Google Scholar] [CrossRef]
- Ali Qamar, O.; Jamil, F.; Hussain, M.; Al-Muhtaseb, A.H.; Inayat, A.; Waris, A.; Akhter, P.; Park, Y.-K. Feasibility-to-Applications of Value-Added Products from Biomass: Current Trends, Challenges, and Prospects. Chem. Eng. J. 2023, 454, 140240. [Google Scholar] [CrossRef]
- Sajid, M.; Farooq, U.; Bary, G.; Azim, M.M.; Zhao, X. Sustainable Production of Levulinic Acid and Its Derivatives for Fuel Additives and Chemicals: Progress, Challenges, and Prospects. Green Chem. 2021, 23, 9198–9238. [Google Scholar] [CrossRef]
- Wu, L.; Wei, W.; Chen, Z.; Chen, X.; Ni, B.-J. Long-Chain Alcohol Production in Open Culture Anaerobic Fermentation. Chem. Eng. J. 2023, 452, 139225. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A Review on Ammonia, Ammonia-Hydrogen and Ammonia-Methane Fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Žula, M.; Grilc, M.; Likozar, B. Hydrocracking, Hydrogenation and Hydro-Deoxygenation of Fatty Acids, Esters and Glycerides: Mechanisms, Kinetics and Transport Phenomena. Chem. Eng. J. 2022, 444, 136564. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent Advances in Carbon Capture Storage and Utilisation Technologies: A Review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Kwon, G.; Cho, D.-W.; Kwon, E.E.; Rinklebe, J.; Wang, H.; Song, H. Beneficial Use of Fe-Impregnated Bentonite as a Catalyst for Pyrolysis of Grass Cut into Syngas, Bio-Oil and Biochar. Chem. Eng. J. 2022, 448, 137502. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Jiang, S.; Huang, H. Nitrogen in Bio-Oil Produced from Hydrothermal Liquefaction of Biomass: A Review. Chem. Eng. J. 2020, 401, 126030. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [Green Version]
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An Overview of the Environmental Pollution and Health Effects Associated with Waste Landfilling and Open Dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536. [Google Scholar] [CrossRef]
- Lauri, P.; Havlík, P.; Kindermann, G.; Forsell, N.; Böttcher, H.; Obersteiner, M. Woody Biomass Energy Potential in 2050. Energy Policy 2014, 66, 19–31. [Google Scholar] [CrossRef]
- IEA. International Energy Association Database; IEA: Paris, France, 2013. [Google Scholar]
- Berndes, G.; Hoogwijk, M.; van den Broek, R. The Contribution of Biomass in the Future Global Energy Supply: A Review of 17 Studies. Biomass Bioenergy 2003, 25, 1–28. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Bellevrat, E.; Kitous, A.; Isaac, M. Bio-Energy Use and Low Stabilization Scenarios. Energy J. 2010, 31, 193–221. [Google Scholar] [CrossRef] [Green Version]
- Offermann, R.; Seidenberger, T.; Thrän, D.; Kaltschmitt, M.; Zinoviev, S.; Miertus, S. Assessment of Global Bioenergy Potentials. Mitig. Adapt. Strateg. Glob Chang. 2011, 16, 103–115. [Google Scholar] [CrossRef]
- Durak, H.; Genel, S.; Durak, E.D.; Özçimen, D.; Koçer, A.T. Hydrothermal Liquefaction Process of Ammi Visnaga and a New Approach for Recycling of the Waste Process Water: Cultivation of Algae and Fungi. Biomass Convers. Biorefin. 2022, 1–17. [Google Scholar] [CrossRef]
- Çolak, U.; Durak, H.; Genel, S. Hydrothermal Liquefaction of Syrian Mesquite (Prosopis Farcta): Effects of Operating Parameters on Product Yields and Characterization by Different Analysis Methods. J. Supercrit. Fluids 2018, 140, 53–61. [Google Scholar] [CrossRef]
- Aysu, T.; Durak, H. Bio-Oil Production via Catalytic Supercritical Liquefaction of Syrian Mesquite (Prosopis Farcta). J. Supercrit. Fluids 2016, 109, 26–34. [Google Scholar] [CrossRef]
- Nazari, L.; Yuan, Z.; Souzanchi, S.; Ray, M.B.; Xu, C. (Charles) Hydrothermal Liquefaction of Woody Biomass in Hot-Compressed Water: Catalyst Screening and Comprehensive Characterization of Bio-Crude Oils. Fuel 2015, 162, 74–83. [Google Scholar] [CrossRef]
- Ding, X.; Mahadevan Subramanya, S.; Fang, T.; Guo, Y.; Savage, P.E. Effects of Potassium Phosphates on Hydrothermal Liquefaction of Triglyceride, Protein, and Polysaccharide. Energy Fuels 2020, 34, 15313–15321. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Liu, S.; Feng, L. Direct Hydrothermal Liquefaction of Undried Macroalgae Enteromorpha Prolifera Using Acid Catalysts. Energy Convers. Manag. 2014, 87, 938–945. [Google Scholar] [CrossRef]
- Biller, P.; Riley, R.; Ross, A.B. Catalytic Hydrothermal Processing of Microalgae: Decomposition and Upgrading of Lipids. Bioresour. Technol. 2011, 102, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Seehar, T.H.; Toor, S.S.; Shah, A.A.; Pedersen, T.H.; Rosendahl, L.A. Biocrude Production from Wheat Straw at Sub and Supercritical Hydrothermal Liquefaction. Energies 2020, 13, 3114. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. Hydrothermal Liquefaction of Beech Wood Using a Natural Calcium Borate Mineral. J. Supercrit. Fluids 2012, 72, 134–139. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Wang, Z.; Liu, B. Catalytic Hydrothermal Liquefaction of Lignin for Production of Aromatic Hydrocarbon over Metal Supported Mesoporous Catalyst. Bioresour. Technol. 2021, 323, 124569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of Solid Biochar Fuel from Waste Biomass by Hydrothermal Carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. Strength, Storage, and Combustion Characteristics of Densified Lignocellulosic Biomass Produced via Torrefaction and Hydrothermal Carbonization. Appl. Energy 2014, 135, 182–191. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal Carbonization of Lignocellulosic Biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef]
- Cao, X.; Ro, K.S.; Libra, J.A.; Kammann, C.I.; Lima, I.; Berge, N.; Li, L.; Li, Y.; Chen, N.; Yang, J.; et al. Effects of Biomass Types and Carbonization Conditions on the Chemical Characteristics of Hydrochars. J. Agric. Food Chem. 2013, 61, 9401–9411. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Wang, J.; Li, X.; Cheng, J.; Yang, H.; Chen, H. Effect of Residence Time on Chemical and Structural Properties of Hydrochar Obtained by Hydrothermal Carbonization of Water Hyacinth. Energy 2013, 58, 376–383. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.; Sermyagina, E.; Saari, J.; Silva de Jesus, M.; Cardoso, M.; Matheus de Almeida, G.; Vakkilainen, E. Hydrothermal Carbonization of Lignocellulosic Agro-Forest Based Biomass Residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- He, Q.; Cheng, C.; Raheem, A.; Ding, L.; Shiung Lam, S.; Yu, G. Effect of Hydrothermal Carbonization on Woody Biomass: From Structure to Reactivity. Fuel 2022, 330, 125586. [Google Scholar] [CrossRef]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal Carbonization for the Preparation of Hydrochars from Glucose, Cellulose, Chitin, Chitosan and Wood Chips via Low-Temperature and Their Characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Luthfi, N.; Nakano, K.; Fukushima, T.; Takisawa, K. Effects of Potassium on Hydrothermal Carbonization of Sorghum Bagasse. Bioresour. Bioprocess 2023, 10, 24. [Google Scholar] [CrossRef]
- Kazimierski, P.; Hercel, P.; Suchocki, T.; Smoliński, J.; Pladzyk, A.; Kardaś, D.; Łuczak, J.; Januszewicz, K. Pyrolysis of Pruning Residues from Various Types of Orchards and Pretreatment for Energetic Use of Biochar. Materials 2021, 14, 2969. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Sivakumar, S.; Joshuva, A.; Deenadayalan, G.; Vishnuvardhan, R. Bio-Fuel Production from Martynia Annua L. Seeds Using Slow Pyrolysis Reactor and Its Effects on Diesel Engine Performance, Combustion and Emission Characteristics. Energy 2021, 217, 119327. [Google Scholar] [CrossRef]
- Durak, H.; Genel, S.; Tunç, M. Pyrolysis of Black Cumin Seed: Significance of Catalyst and Temperature Product Yields and Chromatographic Characterization. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 331–350. [Google Scholar] [CrossRef]
- Aysu, T.; Durak, H.; Güner, S.; Bengü, A.Ş.; Esim, N. Bio-Oil Production via Catalytic Pyrolysis of Anchusa Azurea: Effects of Operating Conditions on Product Yields and Chromatographic Characterization. Bioresour. Technol. 2016, 205, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Aysu, T.; Durak, H. Pyrolysis of Giant Mullein (Verbascum Thapsus L.) in a Fixed-Bed Reactor: Effects of Pyrolysis Parameters on Product Yields and Character. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 661–669. [Google Scholar] [CrossRef]
- Alayont, Ş.; Kayan, D.B.; Durak, H.; Alayont, E.K.; Genel, S. The Role of Acidic, Alkaline and Hydrothermal Pretreatment on Pyrolysis of Wild Mustard (Sinapis Arvensis) on the Properties of Bio-Oil and Bio-Char. Bioresour. Technol. Rep. 2022, 17, 100980. [Google Scholar] [CrossRef]
- Amer, M.W.; Aljariri Alhesan, J.S.; Ibrahim, S.; Qussay, G.; Marshall, M.; Al-Ayed, O.S. Potential Use of Corn Leaf Waste for Biofuel Production in Jordan (Physio-Chemical Study). Energy 2021, 214, 118863. [Google Scholar] [CrossRef]
- Kakku, S.; Naidu, S.; Bhatt, M.; Chakinala, A.G.; Joshi, J.; Gautam, S.; Mohanty, K.; Kataria, G.; Sharma, A. Pyrolytic Conversion of Agricultural Residue Using Continuous Auger Reactor for Resource Recovery. J. Anal. Appl. Pyrolysis 2023, 171, 105951. [Google Scholar] [CrossRef]
- Boscagli, C.; Raffelt, K.; Grunwaldt, J.-D. Reactivity of Platform Molecules in Pyrolysis Oil and in Water during Hydrotreatment over Nickel and Ruthenium Catalysts. Biomass Bioenergy 2017, 106, 63–73. [Google Scholar] [CrossRef]
- Rueangsan, K.; Heman, A.; Kraisoda, P.; Tasarod, H.; Duanguppama, K.; Trisupakitti, S.; Morris, J. Bio-Oil Production via Fast Pyrolysis of Cassava Residues Combined with Ethanol and Volcanic Rock in a Free-Fall Reactor. Cogent Eng. 2023, 10, 2156054. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of Agricultural Waste Biomass towards Production of Gas Fuel and High-Quality Char: Experimental and Numerical Investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Sun, H.; Feng, D.; Sun, S.; Zhao, Y.; Zhang, L.; Chang, G.; Guo, Q.; Wu, J.; Qin, Y. Thermal Evolution of Gas-Liquid-Solid Products and Migration Regulation of C/H/O Elements during Biomass Pyrolysis. J. Anal. Appl. Pyrolysis 2021, 156, 105128. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Nahil, M.A.; Williams, P.T. Pyrolysis-Catalytic Hydrogenation of Cellulose-Hemicellulose-Lignin and Biomass Agricultural Wastes for Synthetic Natural Gas Production. J. Anal. Appl. Pyrolysis 2020, 145, 104753. [Google Scholar] [CrossRef]
- Iminabo, M.; Yip, A.C.K.; Iminabo, J.T.; Pang, S. Application of MgO-Titanomagnetite Mixture in High-Temperature Catalytic Pyrolysis of Radiata Pine. Biomass Convers. Biorefin. 2023, 1–15. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Z.; Chen, G. Catalytic Gasification Characteristics of Cellulose, Hemicellulose and Lignin. Renew Energy 2018, 121, 559–567. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F. Effects of Oxygen and Steam Equivalence Ratios on Updraft Gasification of Biomass. Energies 2021, 14, 2675. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Huang, J.; Williams, P.T. Pyrolysis/Gasification of Cellulose, Hemicellulose and Lignin for Hydrogen Production in the Presence of Various Nickel-Based Catalysts. Fuel 2013, 106, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Bandara, J.C.; Jaiswal, R.; Nielsen, H.K.; Moldestad, B.M.E.; Eikeland, M.S. Air Gasification of Wood Chips, Wood Pellets and Grass Pellets in a Bubbling Fluidized Bed Reactor. Energy 2021, 233, 121149. [Google Scholar] [CrossRef]
- Maglinao, A.L.; Capareda, S.C.; Nam, H. Fluidized Bed Gasification of High Tonnage Sorghum, Cotton Gin Trash and Beef Cattle Manure: Evaluation of Synthesis Gas Production. Energy Convers. Manag. 2015, 105, 578–587. [Google Scholar] [CrossRef]
- Mansur, F.Z.; Faizal, C.K.M.; Samad, N.A.F.A.; Atnaw, S.M.; Sulaiman, S.A. Gasification Performance of Sawdust, Pelletized Sawdust and Sub-Bituminous Coal in a Downdraft Gasifier. SN Appl. Sci. 2020, 2, 1543. [Google Scholar] [CrossRef]
- Chen, P.; Xie, Q.; Addy, M.; Zhou, W.; Liu, Y.; Wang, Y.; Cheng, Y.; Li, K.; Ruan, R. Utilization of Municipal Solid and Liquid Wastes for Bioenergy and Bioproducts Production. Bioresour. Technol. 2016, 215, 163–172. [Google Scholar] [CrossRef] [Green Version]
- EPA, U.S. Advancing Sustainable Materials Management: 2014 Fact Sheet; United States Environmental Protection Agency, Office of Land and Emergency: Washington, DC, USA, 2015.
- EPA, U.S. Clean Watersheds Needs Survey (CWNS) 2012 Report to Congress; United States Environmental Protection Agency: Washington, DC, USA, 2016.
- EPRI, U.S. Electricity Consumption for Water Supply & Treatment—The Next Half Century; EPRI: Palo Alto, CA, USA, 2013. [Google Scholar]
- EPA, U.S. Inventory of US Greenhouse Gas Emissions and Sinks: 1990–2012; The Air Pollution Consultant: New York, NY, USA, 2014; Volume 24, pp. 1_17–1_22. [Google Scholar]
- Kohansal, K.; Toor, S.; Sharma, K.; Chand, R.; Rosendahl, L.; Pedersen, T.H. Hydrothermal Liquefaction of Pre-Treated Municipal Solid Waste (Biopulp) with Recirculation of Concentrated Aqueous Phase. Biomass Bioenergy 2021, 148, 106032. [Google Scholar] [CrossRef]
- Katakojwala, R.; Kopperi, H.; Kumar, S.; Venkata Mohan, S. Hydrothermal Liquefaction of Biogenic Municipal Solid Waste under Reduced H2 Atmosphere in Biorefinery Format. Bioresour. Technol. 2020, 310, 123369. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, Y.-Y.; Liu, H.-C.; Baroutian, S. Optimization of Food Waste Hydrothermal Liquefaction by a Two-Step Process in Association with a Double Analysis. Energy 2020, 199, 117438. [Google Scholar] [CrossRef]
- Aierzhati, A.; Stablein, M.J.; Wu, N.E.; Kuo, C.-T.; Si, B.; Kang, X.; Zhang, Y. Experimental and Model Enhancement of Food Waste Hydrothermal Liquefaction with Combined Effects of Biochemical Composition and Reaction Conditions. Bioresour. Technol. 2019, 284, 139–147. [Google Scholar] [CrossRef]
- Maag, A.R.; Paulsen, A.D.; Amundsen, T.J.; Yelvington, P.E.; Tompsett, G.A.; Timko, M.T. Catalytic Hydrothermal Liquefaction of Food Waste Using CeZrOx. Energies 2018, 11, 564. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Zhang, Z.; Ge, C.; Liu, W.; Tang, Y.; Zhuang, X.; Qiu, R. Synergistic Effect of Hydrothermal Co-Carbonization of Sewage Sludge with Fruit and Agricultural Wastes on Hydrochar Fuel Quality and Combustion Behavior. Waste Manag. 2019, 100, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Vardon, D.R.; Sharma, B.K.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical Properties of Biocrude Oil from the Hydrothermal Liquefaction of Spirulina Algae, Swine Manure, and Digested Anaerobic Sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Prestigiacomo, C.; Costa, P.; Pinto, F.; Schiavo, B.; Siragusa, A.; Scialdone, O.; Galia, A. Sewage Sludge as Cheap Alternative to Microalgae as Feedstock of Catalytic Hydrothermal Liquefaction Processes. J. Supercrit. Fluids 2019, 143, 251–258. [Google Scholar] [CrossRef]
- Shao, Y.; Long, Y.; Zhou, Y.; Jin, Z.; Zhou, D.; Shen, D. 5-Hydroxymethylfurfural Production from Watermelon Peel by Microwave Hydrothermal Liquefaction. Energy 2019, 174, 198–205. [Google Scholar] [CrossRef]
- Minowa, T.; Murakami, M.; Dote, Y.; Ogi, T.; Yokoyama, S. Oil Production from Garbage by Thermochemical Liquefaction. Biomass Bioenergy 1995, 8, 117–120. [Google Scholar] [CrossRef]
- Śliz, M.; Czerwińska, K.; Magdziarz, A.; Lombardi, L.; Wilk, M. Hydrothermal Carbonization of the Wet Fraction from Mixed Municipal Solid Waste: A Fuel and Structural Analysis of Hydrochars. Energies 2022, 15, 6708. [Google Scholar] [CrossRef]
- Śliz, M.; Tuci, F.; Czerwińska, K.; Fabrizi, S.; Lombardi, L.; Wilk, M. Hydrothermal Carbonization of the Wet Fraction from Mixed Municipal Solid Waste: Hydrochar Characteristics and Energy Balance. Waste Manag. 2022, 151, 39–48. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal Carbonization of Sewage Sludge for Energy Production with Coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Reza, M.T.; Coronella, C.; Holtman, K.M.; Franqui-Villanueva, D.; Poulson, S.R. Hydrothermal Carbonization of Autoclaved Municipal Solid Waste Pulp and Anaerobically Treated Pulp Digestate. ACS Sustain. Chem. Eng. 2016, 4, 3649–3658. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The Properties and Combustion Behaviors of Hydrochars Derived from Co-Hydrothermal Carbonization of Sewage Sludge and Food Waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Khoury, O.; Zohar, M.; Poverenov, E.; Darzi, R.; Laor, Y.; Posmanik, R. Hydrothermal Carbonization of Sewage Sludge Coupled with Anaerobic Digestion: Integrated Approach for Sludge Management and Energy Recycling. Energy Convers. Manag. 2020, 224, 113353. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal Carbonisation of Sewage Sludge: Effect of Process Conditions on Product Characteristics and Methane Production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A New Method Combining Hydrothermal Carbonization and Mechanical Compression In-Situ for Sewage Sludge Dewatering: Bench-Scale Verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Hejna, M.; Świechowski, K.; Rasaq, W.A.; Białowiec, A. Study on the Effect of Hydrothermal Carbonization Parameters on Fuel Properties of Chicken Manure Hydrochar. Materials 2022, 15, 5564. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal Carbonization of Anaerobically Digested Sludge for Solid Fuel Production and Energy Recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Velghe, I.; Carleer, R.; Yperman, J.; Schreurs, S. Study of the Pyrolysis of Municipal Solid Waste for the Production of Valuable Products. J. Anal. Appl. Pyrolysis 2011, 92, 366–375. [Google Scholar] [CrossRef]
- Ateş, F.; Miskolczi, N.; Borsodi, N. Comparision of Real Waste (MSW and MPW) Pyrolysis in Batch Reactor over Different Catalysts. Part I: Product Yields, Gas and Pyrolysis Oil Properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Miskolczi, N.; Ateş, F.; Borsodi, N. Comparison of Real Waste (MSW and MPW) Pyrolysis in Batch Reactor over Different Catalysts. Part II: Contaminants, Char and Pyrolysis Oil Properties. Bioresour. Technol. 2013, 144, 370–379. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Zhong, D.; Zhang, B.; Qian, X. Pyrolysis of Municipal Solid Waste in a Fluidized Bed for Producing Valuable Pyrolytic Oils. Clean Technol. Environ. Policy 2016, 18, 1111–1121. [Google Scholar] [CrossRef]
- Syamsiro, M.; Saptoadi, H.; Norsujianto, T.; Noviasri, P.; Cheng, S.; Alimuddin, Z.; Yoshikawa, K. Fuel Oil Production from Municipal Plastic Wastes in Sequential Pyrolysis and Catalytic Reforming Reactors. Energy Procedia 2014, 47, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Gandidi, I.M.; Susila, M.D.; Mustofa, A.; Pambudi, N.A. Thermal—Catalytic Cracking of Real MSW into Bio-Crude Oil. J. Energy Inst. 2018, 91, 304–310. [Google Scholar] [CrossRef]
- Sotoudehnia, F.; Baba Rabiu, A.; Alayat, A.; McDonald, A.G. Characterization of Bio-Oil and Biochar from Pyrolysis of Waste Corrugated Cardboard. J. Anal. Appl. Pyrolysis 2020, 145, 104722. [Google Scholar] [CrossRef]
- Tokmurzin, D.; Kuspangaliyeva, B.; Aimbetov, B.; Abylkhani, B.; Inglezakis, V.; Anthony, E.J.; Sarbassov, Y. Characterization of Solid Char Produced from Pyrolysis of the Organic Fraction of Municipal Solid Waste, High Volatile Coal and Their Blends. Energy 2020, 191, 116562. [Google Scholar] [CrossRef]
- Wang, N.; Qian, K.; Chen, D.; Zhao, H.; Yin, L. Upgrading Gas and Oil Products of the Municipal Solid Waste Pyrolysis Process by Exploiting In-Situ Interactions between the Volatile Compounds and the Char. Waste Manag. 2020, 102, 380–390. [Google Scholar] [CrossRef]
- Tang, F.; Yu, Z.; Li, Y.; Chen, L.; Ma, X. Catalytic Co-Pyrolysis Behaviors, Product Characteristics and Kinetics of Rural Solid Waste and Chlorella Vulgaris. Bioresour. Technol. 2020, 299, 122636. [Google Scholar] [CrossRef]
- Wang, N.; Chen, D.; Arena, U.; He, P. Hot Char-Catalytic Reforming of Volatiles from MSW Pyrolysis. Appl. Energy 2017, 191, 111–124. [Google Scholar] [CrossRef]
- Tursunov, O. A Comparison of Catalysts Zeolite and Calcined Dolomite for Gas Production from Pyrolysis of Municipal Solid Waste (MSW). Ecol. Eng. 2014, 69, 237–243. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Liu, S.; Hu, Z.; Guo, X.; Luo, S.; Yang, F. Syngas Production from Pyrolysis of Municipal Solid Waste (MSW) with Dolomite as Downstream Catalysts. J. Anal. Appl. Pyrolysis 2010, 87, 181–187. [Google Scholar] [CrossRef]
- Gao, N.; Sipra, A.T.; Quan, C. Thermogravimetric Analysis and Pyrolysis Product Characterization of Municipal Solid Waste Using Sludge Fly Ash as Additive. Fuel 2020, 281, 118572. [Google Scholar] [CrossRef]
- He, M.; Hu, Z.; Xiao, B.; Li, J.; Guo, X.; Luo, S.; Yang, F.; Feng, Y.; Yang, G.; Liu, S. Hydrogen-Rich Gas from Catalytic Steam Gasification of Municipal Solid Waste (MSW): Influence of Catalyst and Temperature on Yield and Product Composition. Int. J. Hydrogen Energy 2009, 34, 195–203. [Google Scholar] [CrossRef]
- Kwak, T.-H.; Lee, S.; Maken, S.; Shin, H.-C.; Park, J.-W.; Yoo, Y.D. A Study of Gasification of Municipal Solid Waste Using a Double Inverse Diffusion Flame Burner. Energy Fuels 2005, 19, 2268–2272. [Google Scholar] [CrossRef]
- Kessas, S.A.; Esteves, T.; Hemati, M. Products Distribution During Sewage Sludge Pyrolysis in a Sand and Olivine Fluidized Bed Reactor: Comparison with Woody Waste. Waste Biomass Valorization 2021, 12, 3459–3484. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S. Effect of Particle Size on Pyrolysis of Single-Component Municipal Solid Waste in Fixed Bed Reactor. Int. J. Hydrogen Energy 2010, 35, 93–97. [Google Scholar] [CrossRef]
- Da Silva Filho, V.F.; Batistella, L.; Alves, J.L.F.; da Silva, J.C.G.; Althoff, C.A.; Moreira, R.d.F.P.M.; José, H.J. Evaluation of Gaseous Emissions from Thermal Conversion of a Mixture of Solid Municipal Waste and Wood Chips in a Pilot-Scale Heat Generator. Renew Energy 2019, 141, 402–410. [Google Scholar] [CrossRef]
- EPA US EPA Plastics: Material-Specific Data. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 10 July 2023).
- PlasticsEurope Plastics Europe The Facts 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 10 July 2023).
- Eur. Com. A European Strategy for Plastics in a Circular Economy. 2018. Available online: https://www.switch-asia.eu/resource/a-european-strategy-for-plastics-in-a-circular-economy/ (accessed on 10 July 2023).
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Hamilton, L.A.; Feit, S. Plastic & Climate: The Hidden Costs of a Plastic Planet; CIEL: Washington, DC, USA, 2019. [Google Scholar]
- Liebmann, B.; Köppel, S.; Königshofer, P.; Bucsics, T.; Reiberger, T.; Schwabl, P. Assessment of Microplastic Concentrations in Human Stool: Final Results of a Prospective Study. In Proceedings of the Conference on Nano and Microplastics in Technical and Freshwater Systems, Ascona, Switzerland, 28–31 October 2018; pp. 28–31. [Google Scholar]
- Sharma, S.; Chatterjee, S. Microplastic Pollution, a Threat to Marine Ecosystem and Human Health: A Short Review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Circular Economy Action Plan. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 10 July 2023).
- Lomwongsopon, P.; Varrone, C. Contribution of Fermentation Technology to Building Blocks for Renewable Plastics. Fermentation 2022, 8, 47. [Google Scholar] [CrossRef]
- Mishra, S.; Zope, V.S.; Goje, A.S. Kinetics and Thermodynamics of Hydrolytic Depolymerization of Poly(Ethylene Terephthalate) at High Pressure and Temperature. J. Appl. Polym. Sci. 2003, 90, 3305–3309. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Chatziavgoustis, A.P.; Achilias, D.S. Poly(Ethylene Terephthalate) Recycling and Recovery of Pure Terephthalic Acid by Alkaline Hydrolysis. Adv. Polym. Technol. 2002, 21, 250–259. [Google Scholar] [CrossRef]
- Sato, O.; Arai, K.; Shirai, M. Hydrolysis of Poly(Ethylene Terephthalate) and Poly(Ethylene 2,6-Naphthalene Dicarboxylate) Using Water at High Temperature: Effect of Proton on Low Ethylene Glycol Yield. Catal. Today 2006, 111, 297–301. [Google Scholar] [CrossRef]
- Tagaya, H.; Katoh, K.; Kadokawa, J.; Chiba, K. Decomposition of Polycarbonate in Subcritical and Supercritical Water. Polym. Degrad. Stab. 1999, 64, 289–292. [Google Scholar] [CrossRef]
- Ikeda, A.; Katoh, K.; Tagaya, H. Monomer Recovery of Waste Plastics by Liquid Phase Decomposition and Polymer Synthesis. J. Mater. Sci. 2008, 43, 2437–2441. [Google Scholar] [CrossRef]
- Watanabe, M.; Matsuo, Y.; Matsushita, T.; Inomata, H.; Miyake, T.; Hironaka, K. Chemical Recycling of Polycarbonate in High Pressure High Temperature Steam at 573 K. Polym. Degrad. Stab. 2009, 94, 2157–2162. [Google Scholar] [CrossRef]
- Jin, H.; Bai, B.; Wei, W.; Chen, Y.; Ge, Z.; Shi, J. Hydrothermal Liquefaction of Polycarbonate (PC) Plastics in Sub-/Supercritical Water and Reaction Pathway Exploration. ACS Sustain. Chem. Eng. 2020, 8, 7039–7050. [Google Scholar] [CrossRef]
- Iwaya, T.; Sasaki, M.; Goto, M. Kinetic Analysis for Hydrothermal Depolymerization of Nylon 6. Polym. Degrad. Stab. 2006, 91, 1989–1995. [Google Scholar] [CrossRef]
- Dai, Z.; Hatano, B.; Kadokawa, J.; Tagaya, H. Effect of Diaminotoluene on the Decomposition of Polyurethane Foam Waste in Superheated Water. Polym. Degrad. Stab. 2002, 76, 179–184. [Google Scholar] [CrossRef]
- Watanabe, M.; Hirakoso, H.; Sawamoto, S.; Adschiri, T.; Arai, K. Polyethylene Conversion in Supercritical Water. J. Supercrit. Fluids 1998, 13, 247–252. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jin, K.; Linda Wang, N.-H. Use of Supercritical Water for the Liquefaction of Polypropylene into Oil. ACS Sustain. Chem. Eng. 2019, 7, 3749–3758. [Google Scholar] [CrossRef]
- Jin, K.; Vozka, P.; Gentilcore, C.; Kilaz, G.; Wang, N.-H.L. Low-Pressure Hydrothermal Processing of Mixed Polyolefin Wastes into Clean Fuels. Fuel 2021, 294, 120505. [Google Scholar] [CrossRef]
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Hydrothermal Decomposition of Polyethylene Waste to Hydrocarbons Rich Oil. J. Supercrit. Fluids 2021, 169, 105136. [Google Scholar] [CrossRef]
- Torres-Zapata, T.; Lozano-Martinez, P.; Martinez-Lorenzo, M.V.; Buey, R.M.; Martin-Sanchez, N. Hydrothermally Processed Polyethylene as Starting Point for Fermentative Production of Triglycerides. J. Environ. Chem. Eng. 2022, 10, 108683. [Google Scholar] [CrossRef]
- Kwak, H.; Shin, H.-Y.; Bae, S.-Y.; Kumazawa, H. Characteristics and Kinetics of Degradation of Polystyrene in Supercritical Water. J. Appl. Polym. Sci. 2006, 101, 695–700. [Google Scholar] [CrossRef]
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Wei, W.; Cao, W. Experimental Investigation on Liquefaction of Plastic Waste to Oil in Supercritical Water. Waste Manag. 2019, 89, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Dos Passos, J.S.; Glasius, M.; Biller, P. Screening of Common Synthetic Polymers for Depolymerization by Subcritical Hydrothermal Liquefaction. Process Saf. Environ. Prot. 2020, 139, 371–379. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Hydrothermal Carbonization (HTC) of Marine Plastic Debris. Fuel 2019, 257, 116033. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X. A New Approach to Transforming PVC Waste into Energy via Combined Hydrothermal Carbonization and Fast Pyrolysis. Energy 2017, 141, 1156–1165. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.-D. Hydrothermal Carbonization of Poly(Vinyl Chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xia, Y.; Zhan, L.; Xie, B.; Gao, B.; Wang, J. Hydrothermal Treatment of E-Waste Plastics for Tertiary Recycling: Product Slate and Decomposition Mechanisms. ACS Sustain. Chem. Eng. 2019, 7, 1464–1473. [Google Scholar] [CrossRef]
- Lu, X.; Ma, X.; Chen, X.; Yao, Z.; Zhang, C. Co-Hydrothermal Carbonization of Polyvinyl Chloride and Corncob for Clean Solid Fuel Production. Bioresour. Technol. 2020, 301, 122763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Li, A. Co-Hydrothermal Carbonization of Lignocellulosic Biomass and Waste Polyvinyl Chloride for High-Quality Solid Fuel Production: Hydrochar Properties and Its Combustion and Pyrolysis Behaviors. Bioresour. Technol. 2019, 294, 122113. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, S.; Ge, S.; Chen, X.; Ge, X.; Chen, M. Hydrothermal Carbonization of Medical Wastes and Lignocellulosic Biomass for Solid Fuel Production from Lab-Scale to Pilot-Scale. Energy 2017, 118, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Reza, M.T. Evaluation of Fuel and Combustion Properties of Hydrochar Derived from Co-Hydrothermal Carbonization of Biomass and Plastic. Biomass Bioenergy 2023, 172, 106750. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, R.; Zhang, D.; Gao, Y.; Xiong, M.; Chen, W. Co-Hydrothermal Carbonization of Cotton Textile Waste and Polyvinyl Chloride Waste for the Production of Solid Fuel: Interaction Mechanisms and Combustion Behaviors. J. Clean Prod. 2021, 316, 128306. [Google Scholar] [CrossRef]
- Vasile, C.; Pakdel, H.; Mihai, B.; Onu, P.; Darie, H.; Ciocâlteu, S. Thermal and Catalytic Decomposition of Mixed Plastics. J. Anal. Appl. Pyrolysis 2001, 57, 287–303. [Google Scholar] [CrossRef]
- Donaj, P.J.; Kaminsky, W.; Buzeto, F.; Yang, W. Pyrolysis of Polyolefins for Increasing the Yield of Monomers’ Recovery. Waste Manag. 2012, 32, 840–846. [Google Scholar] [CrossRef]
- Escola, J.M.; Aguado, J.; Serrano, D.P.; García, A.; Peral, A.; Briones, L.; Calvo, R.; Fernandez, E. Catalytic Hydroreforming of the Polyethylene Thermal Cracking Oil over Ni Supported Hierarchical Zeolites and Mesostructured Aluminosilicates. Appl. Catal. B 2011, 106, 405–415. [Google Scholar] [CrossRef]
- Huang, W.-C.; Huang, M.-S.; Huang, C.-F.; Chen, C.-C.; Ou, K.-L. Thermochemical Conversion of Polymer Wastes into Hydrocarbon Fuels over Various Fluidizing Cracking Catalysts. Fuel 2010, 89, 2305–2316. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, D.; Lei, H.; Villota, E.; Ruan, R. Jet Fuel Production from Waste Plastics via Catalytic Pyrolysis with Activated Carbons. Appl. Energy 2019, 251, 113337. [Google Scholar] [CrossRef]
- Grause, G.; Matsumoto, S.; Kameda, T.; Yoshioka, T. Pyrolysis of Mixed Plastics in a Fluidized Bed of Hard Burnt Lime. Ind. Eng. Chem. Res. 2011, 50, 5459–5466. [Google Scholar] [CrossRef]
- Li, K.; Lei, J.; Yuan, G.; Weerachanchai, P.; Wang, J.-Y.; Zhao, J.; Yang, Y. Fe-, Ti-, Zr- and Al-Pillared Clays for Efficient Catalytic Pyrolysis of Mixed Plastics. Chem. Eng. J. 2017, 317, 800–809. [Google Scholar] [CrossRef]
- Areeprasert, C.; Khaobang, C. Pyrolysis and Catalytic Reforming of ABS/PC and PCB Using Biochar and e-Waste Char as Alternative Green Catalysts for Oil and Metal Recovery. Fuel Process. Technol. 2018, 182, 26–36. [Google Scholar] [CrossRef]
- Li, K.; Lee, S.W.; Yuan, G.; Lei, J.; Lin, S.; Weerachanchai, P.; Yang, Y.; Wang, J.-Y. Investigation into the Catalytic Activity of Microporous and Mesoporous Catalysts in the Pyrolysis of Waste Polyethylene and Polypropylene Mixture. Energies 2016, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Huang, Q.; Meng, X.; Chi, Y.; Yan, J. Catalytic Pyrolysis of Waste Polyethylene into Aromatics by H3PO4-Activated Carbon. Energy Fuels 2018, 32, 9772–9781. [Google Scholar] [CrossRef]
- Al-asadi, M.; Miskolczi, N. High Temperature Pyrolysis of Municipal Plastic Waste Using Me/Ni/ZSM-5 Catalysts: The Effect of Metal/Nickel Ratio. Energies 2020, 13, 1284. [Google Scholar] [CrossRef] [Green Version]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Xiao, B.; Hu, Z.; Liu, S.; Guo, X.; Luo, S. Syngas Production from Catalytic Gasification of Waste Polyethylene: Influence of Temperature on Gas Yield and Composition. Int. J. Hydrogen Energy 2009, 34, 1342–1348. [Google Scholar] [CrossRef]
- Arena, U.; Zaccariello, L.; Mastellone, M.L. Fluidized Bed Gasification of Waste-Derived Fuels. Waste Manag. 2010, 30, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Saebea, D.; Ruengrit, P.; Arpornwichanop, A.; Patcharavorachot, Y. Gasification of Plastic Waste for Synthesis Gas Production. Energy Rep. 2020, 6, 202–207. [Google Scholar] [CrossRef]
- Arena, U.; Di Gregorio, F. Energy Generation by Air Gasification of Two Industrial Plastic Wastes in a Pilot Scale Fluidized Bed Reactor. Energy 2014, 68, 735–743. [Google Scholar] [CrossRef]
- Zaccariello, L.; Mastellone, M.L. Fluidized-Bed Gasification of Plastic Waste, Wood, and Their Blends with Coal. Energies 2015, 8, 8052–8068. [Google Scholar] [CrossRef] [Green Version]
- Arena, U.; Di Gregorio, F. Fluidized Bed Gasification of Industrial Solid Recovered Fuels. Waste Manag. 2016, 50, 86–92. [Google Scholar] [CrossRef]
- Sancho, J.A.; Aznar, M.P.; Toledo, J.M. Catalytic Air Gasification of Plastic Waste (Polypropylene) in Fluidized Bed. Part I: Use of in-Gasifier Bed Additives. Ind. Eng. Chem. Res. 2008, 47, 1005–1010. [Google Scholar] [CrossRef]
- Kim, J.-W.; Mun, T.-Y.; Kim, J.-O.; Kim, J.-S. Air Gasification of Mixed Plastic Wastes Using a Two-Stage Gasifier for the Production of Producer Gas with Low Tar and a High Caloric Value. Fuel 2011, 90, 2266–2272. [Google Scholar] [CrossRef]
- Cho, M.-H.; Mun, T.-Y.; Kim, J.-S. Production of Low-Tar Producer Gas from Air Gasification of Mixed Plastic Waste in a Two-Stage Gasifier Using Olivine Combined with Activated Carbon. Energy 2013, 58, 688–694. [Google Scholar] [CrossRef]
- Martínez-Lera, S.; Torrico, J.; Pallarés, J.; Gil, A. Design and First Experimental Results of a Bubbling Fluidized Bed for Air Gasification of Plastic Waste. J. Mater. Cycles Waste Manag. 2013, 15, 370–380. [Google Scholar] [CrossRef]
- Lee, J.W.; Yu, T.U.; Lee, J.W.; Moon, J.H.; Jeong, H.J.; Park, S.S.; Yang, W.; Lee, U. Do Gasification of Mixed Plastic Wastes in a Moving-Grate Gasifier and Application of the Producer Gas to a Power Generation Engine. Energy Fuels 2013, 27, 2092–2098. [Google Scholar] [CrossRef]
- Cao, C.; Bian, C.; Wang, G.; Bai, B.; Xie, Y.; Jin, H. Co-Gasification of Plastic Wastes and Soda Lignin in Supercritical Water. Chem. Eng. J. 2020, 388, 124277. [Google Scholar] [CrossRef]
- Cagnetta, G.; Zhang, K.; Zhang, Q.; Huang, J.; Yu, G. Augmented Hydrogen Production by Gasification of Ball Milled Polyethylene with Ca(OH)2 and Ni(OH)2. Front. Environ. Sci. Eng. 2019, 13, 11. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Cui, D.; Zhou, H.; Wu, D.; Pan, S.; Xu, F.; Wang, Z. Co-Hydrothermal Carbonization of Organic Solid Wastes to Hydrochar as Potential Fuel: A Review. Sci. Total Environ. 2022, 850, 158034. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Liu, Z.; Li, Y.; Xu, J.; Li, J.; Liu, B.; Sun, Q. Combustion Characteristics, Kinetic and Thermodynamic Analyses of Hydrochars Derived from Hydrothermal Carbonization of Cattle Manure. J. Environ. Chem. Eng. 2022, 10, 106938. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Xu, B.; Li, C.; Zeng, G. Feedwater PH Affects Phosphorus Transformation during Hydrothermal Carbonization of Sewage Sludge. Bioresour. Technol. 2017, 245, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal Carbonization of Renewable Waste Biomass for Solid Biofuel Production: A Discussion on Process Mechanism, the Influence of Process Parameters, Environmental Performance and Fuel Properties of Hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A Review of the Hydrothermal Carbonization of Biomass Waste for Hydrochar Formation: Process Conditions, Fundamentals, and Physicochemical Properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars Production, Characterization and Application for Wastewater Treatment: A Review. Renew Sustain. Energy Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- WBA. WBA Global Bioenergy Statistics 2018. 2023. Available online: https://www.worldbioenergy.org/uploads/181017%20WBA%20GBS%202018_Summary_hq.pdf (accessed on 10 July 2023).
- Fan, Y.; Hornung, U.; Dahmen, N.; Kruse, A. Hydrothermal Liquefaction of Protein-Containing Biomass: Study of Model Compounds for Maillard Reactions. Biomass Convers. Biorefin. 2018, 8, 909–923. [Google Scholar] [CrossRef]
- Maddi, B.; Panisko, E.; Wietsma, T.; Lemmon, T.; Swita, M.; Albrecht, K.; Howe, D. Quantitative Characterization of Aqueous Byproducts from Hydrothermal Liquefaction of Municipal Wastes, Food Industry Wastes, and Biomass Grown on Waste. ACS Sustain. Chem. Eng. 2017, 5, 2205–2214. [Google Scholar] [CrossRef]
- Saengsuriwong, R.; Onsree, T.; Phromphithak, S.; Tippayawong, N. Conversion of Tobacco Processing Waste to Biocrude Oil via Hydrothermal Liquefaction in a Multiple Batch Reactor. Clean Technol. Environ. Policy 2023, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Bayat, H.; Dehghanizadeh, M.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Hydrothermal Liquefaction of Food Waste: Effect of Process Parameters on Product Yields and Chemistry. Front. Sustain. Food Syst. 2021, 5, 658592. [Google Scholar] [CrossRef]
- Kostyukevich, Y.; Vlaskin, M.; Borisova, L.; Zherebker, A.; Perminova, I.; Kononikhin, A.; Popov, I.; Nikolaev, E. Investigation of Bio-Oil Produced by Hydrothermal Liquefaction of Food Waste Using Ultrahigh Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Eur. J. Mass Spectrom. 2018, 24, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Motavaf, B.; Savage, P.E. Effect of Process Variables on Food Waste Valorization via Hydrothermal Liquefaction. ACS EST Eng. 2021, 1, 363–374. [Google Scholar] [CrossRef]
- Aydıncak, K.; Yumak, T.; Sınağ, A.; Esen, B. Synthesis and Characterization of Carbonaceous Materials from Saccharides (Glucose and Lactose) and Two Waste Biomasses by Hydrothermal Carbonization. Ind. Eng. Chem. Res. 2012, 51, 9145–9152. [Google Scholar] [CrossRef]
- Mazaheri, H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Subcritical Water Liquefaction of Oil Palm Fruit Press Fiber for the Production of Bio-Oil: Effect of Catalysts. Bioresour. Technol. 2010, 101, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Quitain, A.T.; Sato, N.; Daimon, H.; Fujie, K. Production of Valuable Materials by Hydrothermal Treatment of Shrimp Shells. Ind. Eng. Chem. Res. 2001, 40, 5885–5888. [Google Scholar] [CrossRef]
- Hadhoum, L.; Balistrou, M.; Burnens, G.; Loubar, K.; Tazerout, M. Hydrothermal Liquefaction of Oil Mill Wastewater for Bio-Oil Production in Subcritical Conditions. Bioresour. Technol. 2016, 218, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Idowu, I.; Li, L.; Flora, J.R.V.; Pellechia, P.J.; Darko, S.A.; Ro, K.S.; Berge, N.D. Hydrothermal Carbonization of Food Waste for Nutrient Recovery and Reuse. Waste Manag. 2017, 69, 480–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauline, A.L.; Joseph, K. Hydrothermal Carbonization of Crude Oil Sludge—Characterization of Hydrochar and Hydrothermal Liquor. Process Saf. Environ. Prot. 2021, 154, 89–96. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal Carbonization of Oily Sludge for Solid Fuel Recovery—Investigation of Chemical Characteristics and Combustion Behaviour. J. Anal. Appl. Pyrolysis 2021, 157, 105235. [Google Scholar] [CrossRef]
- Atallah, E.; Kwapinski, W.; Ahmad, M.N.; Leahy, J.J.; Al-Muhtaseb, A.H.; Zeaiter, J. Hydrothermal Carbonization of Olive Mill Wastewater: Liquid Phase Product Analysis. J. Environ. Chem. Eng. 2019, 7, 102833. [Google Scholar] [CrossRef]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, E.C.; Weiss-Hortala, E.; Fiori, L. Agro-Industrial Waste to Solid Biofuel through Hydrothermal Carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Benavente, V.; Calabuig, E.; Fullana, A. Upgrading of Moist Agro-Industrial Wastes by Hydrothermal Carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Suárez, L.; Benavente-Ferraces, I.; Plaza, C.; de Pascual-Teresa, S.; Suárez-Ruiz, I.; Centeno, T.A. Hydrothermal Carbonization as a Sustainable Strategy for Integral Valorisation of Apple Waste. Bioresour. Technol. 2020, 309, 123395. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Zhang, S.; Chang, G.; Wang, X.; Zeng, Z. Evaluation on the Enhanced Solid Biofuel from Co-Hydrothermal Carbonization of Pharmaceutical Biowastes with Lignite. Fuel 2022, 318, 123626. [Google Scholar] [CrossRef]
- Lin, Y.; Ge, Y.; Xiao, H.; He, Q.; Wang, W.; Chen, B. Investigation of Hydrothermal Co-Carbonization of Waste Textile with Waste Wood, Waste Paper and Waste Food from Typical Municipal Solid Wastes. Energy 2020, 210, 118606. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, G.; Zhao, P.; Areeprasert, C.; Shen, Y.; Yoshikawa, K.; Xu, G. Hydrothermal Treatment of Antibiotic Mycelial Dreg: More Understanding from Fuel Characteristics. Chem. Eng. J. 2015, 273, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Azzaz, A.A.; Matei Ghimbeu, C.; Jellai, S.; El-Bassi, L.; Jeguirim, M. Olive Mill By-Products Thermochemical Conversion via Hydrothermal Carbonization and Slow Pyrolysis: Detailed Comparison between the Generated Hydrochars and Biochars Characteristics. Processes 2022, 10, 231. [Google Scholar] [CrossRef]
- Demiral, İ.; Şensöz, S. The Effects of Different Catalysts on the Pyrolysis of Industrial Wastes (Olive and Hazelnut Bagasse). Bioresour. Technol. 2008, 99, 8002–8007. [Google Scholar] [CrossRef]
- Tiikma, L.; Tamvelius, H.; Luik, L. Coprocessing of Heavy Shale Oil with Polyethylene Waste. J. Anal. Appl. Pyrolysis 2007, 79, 191–195. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Guimarães, L.; Oliveira, M.; Kille, P.; Ferreira, N.G.C. Thermochemical Conversion Processes as a Path for Sustainability of the Tire Industry: Carbon Black Recovery Potential in a Circular Economy Approach. Clean Technol. 2022, 4, 653–668. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, A.; Jiang, E.; Qin, L. Pyrolysis of Industrial Waste Lignin: Analysis of Product Yields and Character. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 458–464. [Google Scholar] [CrossRef]
- Caglar, A.; Aydinli, B. The Pyrolysis of Industrial Alliaceous Plant Wastes: Illustration of Process and Characterization of Products. Energy Explor. Exploit. 2018, 36, 1692–1707. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhan, S.; Yu, H.; Xue, X.; Hong, N. The Effects of Temperature and Catalysts on the Pyrolysis of Industrial Wastes (Herb Residue). Bioresour. Technol. 2010, 101, 3236–3241. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Lustosa Filho, J.F.; Melo, L.C.A.; de Assis, I.R.; de Oliveira, T.S. Influence of Pyrolysis Temperature and Feedstock on the Properties of Biochars Produced from Agricultural and Industrial Wastes. J. Anal. Appl. Pyrolysis 2020, 149, 104839. [Google Scholar] [CrossRef]
- Önal, E.P.; Uzun, B.B.; Pütün, A.E. Steam Pyrolysis of an Industrial Waste for Bio-Oil Production. Fuel Process. Technol. 2011, 92, 879–885. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Ali, I.; Nasir, S.; Ali Ammar Taqvi, S.; Atabani, A.E.; Chen, W.-H. Assessment of Agro-Industrial Residues for Bioenergy Potential by Investigating Thermo-Kinetic Behavior in a Slow Pyrolysis Process. Fuel 2020, 278, 118259. [Google Scholar] [CrossRef]
- Castillo Santiago, Y.; Pérez, J.F.; Sphaier, L.A. Reaction-Front and Char Characterization from a Palm Kernel Shell—Oil Sludge Mixture under Oxygen Lean Regimes in a Fixed-Bed Gasifier. Fuel 2023, 333, 126402. [Google Scholar] [CrossRef]
- Ge, Z.; Cao, X.; Zha, Z.; Ma, Y.; Zeng, M.; Wu, Y.; Hou, Z.; Zhang, H. Establishment of Correlation between Reaction Kinetics and Carbon Structures in the Char Gasification Process. Carbon Resour. Convers. 2023, 6, 67–75. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Ayol, A. Gasification Performance of Olive Pomace in Updraft and Downdraft Fixed Bed Reactors. Int. J. Hydrogen Energy 2023, 48, 22909–22920. [Google Scholar] [CrossRef]
- Ongen, A.; Ozcan, H.K.; Ozbaş, E.E.; Aydin, S.; Yesildag, I. Co-Gasification of Oily Sludge and Chicken Manure in a Laboratory-Scale Updraft Fixed Bed Gasifier. Clean Technol. Env. Policy 2022, 24, 2229–2239. [Google Scholar] [CrossRef]
- Subramanian, A.S.R.; Gundersen, T.; Adams, T.A. Technoeconomic Analysis of a Waste Tire to Liquefied Synthetic Natural Gas (SNG) Energy System. Energy 2020, 205, 117830. [Google Scholar] [CrossRef]
- Portofino, S.; Donatelli, A.; Iovane, P.; Innella, C.; Civita, R.; Martino, M.; Matera, D.A.; Russo, A.; Cornacchia, G.; Galvagno, S. Steam Gasification of Waste Tyre: Influence of Process Temperature on Yield and Product Composition. Waste Manag. 2013, 33, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-H.; Lan Thao Ngo, T.N.; Chiang, K.-Y. Enhanced Hydrogen Production in Co-Gasification of Sewage Sludge and Industrial Wastewater Sludge by a Pilot-Scale Fluidized Bed Gasifier. Int. J. Hydrogen Energy 2021, 46, 14083–14095. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Chiang, K.-Y. Sewage and Textile Sludge Co-Gasification Using a Lab-Scale Fluidized Bed Gasifier. Int. J. Hydrogen Energy 2022, 47, 40613–40627. [Google Scholar] [CrossRef]
- Vera, D.; Jurado, F.; Margaritis, N.K.; Grammelis, P. Experimental and Economic Study of a Gasification Plant Fuelled with Olive Industry Wastes. Energy Sustain. Dev. 2014, 23, 247–257. [Google Scholar] [CrossRef]
- Yan, J.; Yan, Y.; Lai, J.; Jia, D.; Jiao, Y.; Zhao, X.; Yang, L. Co-Gasification of Municipal Sewage Sludge and Cotton Stalk Enhanced by Metal-Enriched Texture Dyeing Sludge Additives for Syngas Production. Fuel 2023, 341, 127669. [Google Scholar] [CrossRef]
- Wyszyński, Z.; Michalska-Klimczak, B.; Pągowski, K.; Kamińska, S. Biomass as the Main Source of Renewable Energy in Poland. In Proceedings of the International Scientific Conference: Renewable Energy and Energy Efficiency, Jelgava, Latvia, 28–30 May 2012; Latvia University of Agriculture: Jelgava, Latvia, 2012. [Google Scholar]
- Habert, G.; Bouzidi, Y.; Chen, C.; Jullien, A. Development of a Depletion Indicator for Natural Resources Used in Concrete. Resour. Conserv. Recycl. 2010, 54, 364–376. [Google Scholar] [CrossRef]
- Sönnichsen, N. Global Primary Energy Consumption 2000–2021. 2022. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/265598/consumption-of-primary-energy-worldwide/ (accessed on 10 July 2023).
- Sönnichsen, N. Energy Consumption Worldwide from 2000 to 2019, with a Forecast until 2050, by Energy Source. 2022. Available online: https://www-1statista-1com-1s8fui2pq12ae.han3.ue.poznan.pl/statistics/222066/projected-global-energy-consumptionby-source/ (accessed on 10 July 2023).
- Wilson, D.C.; Velis, C.A.; Rodic, L. Integrated Sustainable Waste Management in Developing Countries. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2013, 166, 52–68. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A.; Singh, E.; Kumar, S. Carbonaceous Nanocomposites Derived from Waste Material for Wastewater Treatment. In Biorenewable Nanocomposite Materials, Vol. 2: Desalination and Wastewater Remediation Chapter; American Chemical Society: Washington, DC, USA, 2022; Volume 3, pp. 43–73. [Google Scholar] [CrossRef]
- Dhote, L.; Ganduri, J.; Kumar, S. Evaluation of pyrolysis and gasification of distillery sludge and bio-compost mixed with coal. Fuel 2022, 319, 123750. [Google Scholar] [CrossRef]
- Bombaywala, S.; Mandpe, A.; Paliya, S.; Kumar, S. Antibiotic resistance in the environment: A critical insight on its occurrence, fate, and eco-toxicity. Environ. Sci. Pollut. Res. 2021, 28, 24889–24916. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Bi, X. Life-cycle assessment of transportation biofuels from hydrothermal liquefaction of forest residues in British Columbia. Biotechnol. Biofuels 2018, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Botas, J.A.; Moreno, J.; Espada, J.J.; Serrano, D.P.; Dufour, J. Recycling of used lubricating oil: Evaluation of environmental and energy performance by LCA. Resour. Conserv. Recycl. 2017, 125, 315–323. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Torres-Mayanga, P.C.; Abaide, E.R.; Zabot, G.L.; De Castilhos, F. Hydrothermal carbonization and Liquefaction: Differences, progress, challenges, and opportunities. Bioresour. Technol. 2022, 343, 126084. [Google Scholar] [CrossRef] [PubMed]
- Stobernack, N.; Mayer, F.; Malek, C.; Bhandari, R. Evaluation of the energetic and environmental potential of the hydrothermal carbonization of biowaste: Modeling of the entire process chain. Bioresour. Technol. 2020, 318, 124038. [Google Scholar] [CrossRef]
- Bora, R.R.; Lei, M.; Tester, J.W.; Lehmann, J.; You, F. Life Cycle Assessment and Technoeconomic Analysis of Thermochemical Conversion Technologies Applied to Poultry Litter with Energy and Nutrient Recovery. ACS Sustain. Chem. Eng. 2020, 8, 8436–8447. [Google Scholar] [CrossRef]




| Conversion Process | Feed Stock | Reaction Parameters | Catalyst | HHV | Ref. |
|---|---|---|---|---|---|
| Hydrothermal Liquefaction (HTL) | Ammi visnaga | 250–325 °C, 0–45 min | Cu, W, Fe metal powders | 30.30 MJ/kg | [45] |
| Prosopis farcta | 250–350 °C, 0 min | H3BO3, Na2B4O7·10H2O, NaOH | 19.75–30.02 MJ/kg | [46] | |
| Datura stramonium L. | 250–380 °C, 3.91–23.23 Mpa, 15 min | Colemanite and borax | - | [47] | |
| Birchwood sawdust | 300 °C, 30 min | KOH, FeSO4·7H2O, K2CO3, MgO, synthetic hydrotalcite, colemanite | 26.0–31.3 MJ/kg | [48] | |
| Soybean oil, potato starch, microcrystalline cellulose | 320 °C, 30 min | KH2PO4, K2HPO4, K3PO4 | 13.40–36 MJ/kg | [49] | |
| Macroalgae (Enteromorpha prolifera) | 230–310 °C, 20–100 min | Sulfuric acid and acetic acid | 24.5–32.00 MJ/kg | [50] | |
| Microalgae and cyanobacteria | 350 °C, 250 bar | Na2CO3 and formic acid | 32.3–37.1 MJ/kg | [51] | |
| Wheat straw | 350, 400 °C, 18–35 Mpa, 15 min | K2CO3 | 16.53–35.97 MJ/kg | [52] | |
| Beech wood | 250–350 °C, 0 min | Calcium borate mineral and colemanite | 23.81–27.53 MJ/kg | [53] | |
| Lignin | 250–310 °C | MCM-41, Ni-Al/MCM-41 | 25.4–36.6 MJ/kg | [54] | |
| Hydrothermal Carbonization (HTC) | Coconut fiber and dead eucalyptus leaves | 150–375 °C, 30 min | - | 18.4–30.6 MJ/kg | [55] |
| Miscanthus | 190–260 °C | - | 18.5–25.9 MJ/kg | [56] | |
| Cellulose, lignin, D-xylose, wood meal | 225–265 °C | - | 24–30 MJ/kg | [57] | |
| Corn stalk and Tamarix ramosissima | 250 °C, 580 psi, 4 h | - | 28.4–29.2 MJ/kg | [58] | |
| Bark mulch and sugar beet pulp | 200, 250 °C, 3–20 h | - | - | [59] | |
| Water hyacinth | 240 °C, 30 min–24 h | - | 16.83–20.63 MJ/kg | [60] | |
| Giant bamboo, coffee wood, eucalyptus, and coffee parchment | 180–240 °C, 3 h | - | 24.6–29.2 MJ/kg | [61] | |
| Pinewood and poplar wood | 180–240 °C | - | [62] | ||
| Glucose, cellulose, chitin, chitosan, wood chips | 200 °C, 6 h | - | 13.29–25.45 MJ/kg | [63] | |
| Sorghum bagasse | 250 °C, | - | 26.4–31.9 MJ/kg | [64] | |
| Pyrolysis | Pruning residues | 400–600 °C | - | 23.45–30.53 MJ/kg | [65] |
| Martynia annua L. Seeds | 650 °C, 3 h | - | - | [66] | |
| Black cumin seed | 300–500 °C, | Ca(OH)2, Al2O3, SnCl4·5H2O | 32.44–36.19 MJ/kg | [67] | |
| Anchusea Azurea | 350–550 °C | Ca(OH)2, Na2CO3, ZnCl2, Al2O3 | 17.43–24.18 MJ/kg | [68] | |
| Verbascum thapsus L. | 400–550 °C | Al2O3, ZnO | - | [69] | |
| Wild mustard (Sinapis arvensis) | 350–550 °C | H2SO4, HCI, NaOH, KOH, | 12.35–28.06 MJ/kg | [70] | |
| Corn leaf waste | 300–450 °C | - | 24.4–26.1 MJ/kg | [71] | |
| Agricultural residue | 400–700 °C | - | 21.84–26.64 MJ/kg | [72] | |
| Wheat straw | 340 °C, 1.6 h | Ru/C, NiCu/Al2O3 | 35.1–37.5 MJ/kg | [73] | |
| Cassava residues | 450–500 °C | Volcanic rock | 18.4–19.8 MJ/kg | [74] | |
| Gasification | Oat straw | 300–600 °C | - | - | [75] |
| Corn straw | 500–800 °C | - | - | [76] | |
| Cellulose, hemicellulose (xylan), lignin | 500–800 °C | Ni/Al2O3 | - | [77] | |
| Radiata pine | 850 °C | MgO-Titanomagnetite | - | [78] | |
| Cellulose, hemicellulose, lignin, straw, pine | 1000 °C | Dolomite and Na2CO3 | - | [79] | |
| Wood (eucalyptus), lignin, shells of almond and hazelnut, lignin | 600–1000 °C | - | - | [80] | |
| Cellulose, hemicellulose, lignin | 500–800 °C | Ni-Ca-Al | - | [81] | |
| Wood chips, wood pellets, grass pellets | 650–800 °C | - | - | [82] | |
| Sorghum, cotton gin trash | 730–800 °C | - | - | [83] | |
| Sawdust, pelletized sawdust | 750 °C | - | - | [84] |
| Conversion Process | Feed Stock | Reaction Parameters | Catalyst | HHV | Ref. |
|---|---|---|---|---|---|
| Hydrothermal Liquefaction (HTL) | Biopulp | 350–400 °C | K2CO3 | 37.4–40.7 MJ/kg | [90] |
| Biogenic waste | 200 °C, 100 bar | - | - | [91] | |
| Food waste | 300–350 °C, 0, 30, 60 min | K2CO3 | 25.12 MJ/kg | [92] | |
| Food waste | 280–380 °C, 10–60 min | - | 32.71 MJ/kg | [93] | |
| Food waste | 300 °C, 1 h | Na2CO3, CeZrOx | 24.20–35.60 MJ/kg | [94] | |
| Fruit and agricultural wastes | 220 °C, | - | 21.72 MJ/kg | [95] | |
| Spirulina algae, swine manure, and digested anaerobic sludge | 300 °C, 10–12 Mpa, 30 min | - | 32.0–34.7 MJ/kg | [96] | |
| Sewage sludge and Chlorella vulgaris | 325 °C, 30 min | NiMo/Al2O3, CoMo/Al2O3, activated carbon felt | 38.19 MJ/kg | [97] | |
| Watermelon peel | 130 °C, 6 min | - | 18.86 MJ/kg | [98] | |
| Garbage | 250–340 °C–18 Mpa, 10, 30, 120 min | - | 36 MJ/kg | [99] | |
| Hydrothermal Carbonization (HTC) | Municipal mixed waste | 200–220 °C, 1, 4, 8 h | - | 13.5–18.5 MJ/kg | [100] |
| Mixed municipal solid waste | 180–220 °C, 1, 4, 8 h | - | 18.46 MJ/kg | [101] | |
| Sewage sludge | 250 °C, 8–10 Mpa, 15 min | - | 15.82 MJ/kg | [102] | |
| Municipal solid waste pulp | 200–300 °C, 30–120 min | - | 15.8–21.0 MJ/kg | [103] | |
| Sewage sludge | 180–280 °C, 60 min | - | 10.11–31.50 MJ/kg | [104] | |
| Sewage sludge | 200–300 °C, 30, 60, 120 min | - | 18.2–21.5 MJ/kg | [105] | |
| Sewage sludge | 140–200 °C, 30–240 min | - | 16.66–18.65 MJ/kg | [106] | |
| Sewage sludge | 160–240 °C, 60 min | - | 9.82–11.57 MJ/kg | [107] | |
| Chicken manure | 180–300 °C, 30–180 min | - | 15.5 MJ/kg | [108] | |
| Anaerobically digested sludge | 180–250 °C, 30 min | - | 16.5–22.4 MJ/kg | [109] | |
| Pyrolysis | Municipal solid waste | 450–550 °C | - | 20 MJ/Nm3 | [110] |
| Mixture of plastic, paper, textile, and other organic wastes | 500–600 °C | Y-zeolite, β-zeolite, FCC equilibrium, HZSM-5 | 18.41–50.69 MJ/kg | [111] | |
| Mixture of plastic, paper, textile, and other organic wastes | 500–600 °C | Y-zeolite, b-zeolite, equilibrium FCC, MoO3, Ni-Mo-catalyst, HZSM-5, Al(OH)3 | - | [112] | |
| Mixture of biomass, plastic, paper, and cardboard | 450–550 °C | - | 27.5–32.1 MJ/kg | [113] | |
| Municipal plastic wastes | 450 °C | Commercial Y-zeolite and natural zeolite | - | [114] | |
| Municipal solid waste plastics, biomass, paper, rubber and textile | 400 °C, 60 min | Natural activated zeolite | - | [115] | |
| Corrugated cardboard | 350–450 °C | - | 18–21.7 MJ/kg | [116] | |
| Organic fraction of municipal solid waste | 500–800 °C | - | 17.77–26.97 MJ/kg | [117] | |
| Municipal solid waste | 500–700 °C | - | 10.2–27.0 MJ/kg | [118] | |
| Rural solid waste and Chlorella vulgaris | 500 °C | CaO, MgO, HZSM-5 | 20.13–22.39 MJ/kg | [119] | |
| Gasification | Kitchen garbage, paper, cloth and fiber, plastics, residue | 500–600 °C | - | - | [120] |
| Kitchen garbage, textile, wood, and plastic, glass, ferrous materials | 200–750 °C | Zeolite and calcined dolomite | - | [121] | |
| Kitchen waste, paper, textile, wood, plastic | 750–900 °C | Calcined dolomite | - | [122] | |
| Plastics, paper, other organic wastes (wood, thin foil, vegetable garbage, etc.), textile/synthetic fibres, metal | 500–600 °C | - | - | [111] | |
| Plastics, rubber, wood, cloth | 700 °C | - | - | [123] | |
| MSW | 750–950 °C | Dolomite | - | [124] | |
| MSW | 600–1200 °C | - | 9.31–15.45 MJ/kg | [125] | |
| Sewage sludge (SS) | 700–890 °C | - | - | [126] | |
| MSW | 800 °C | - | - | [127] | |
| Municipal waste and wood chips | 800–1000 °C | - | 23.93 MJ kg | [128] |
| Conversion Process | Feed Stock | Reaction Parameters | Catalyst | HHV | Ref. |
|---|---|---|---|---|---|
| Hydrothermal Liquefaction (HTL) | PET (polyethylene terephthalate) | 100–250 °C, 120–200 °C, 300–420 °C | NaOH and KOH | - | [138,139,140] |
| Polycarbonate (PC) | 230, 430 °C, 130–300 °C, 400 °C | Na2CO3 | - | [141,142,143,144] | |
| Polyamide 6 (PA6) | 300–400 °C, 35 Mpa | - | - | [145] | |
| Polyurethane (PU) | 150–350 °C | Diaminotoluene (TDA) | - | [146] | |
| Polyethylene (PE) | 400–450 °C | - | - | [147] | |
| Polyproplyene | 380–500 °C | - | 48–49 MJ/kg | [148] | |
| Polyolefin | 450 °C, 45–60 min, 23 Mpa | - | - | [149] | |
| Polyethylene (LDPE, PE) and Polyethylene | 380–450 °C, 30–240 min 425–450 °C, 30–180 min | Acetic acid | - | [150,151] | |
| Polystyrene High impact polystyrene (HIPS) | 380–420 °C, 0–60 min 350–550 °C, 5–60 min | - | - | [152,153] | |
| Synthetic polymers | 350 °C, 20 min | KOH | - | [154] | |
| Hydrothermal Carbonization (HTC) | Marine plastic debris | 200–300 °C 3 h | - | - | [155] |
| PVC waste | 200–260 °C, 60 min | - | 17.43–36.87 MJ/kg | [156] | |
| Poly(vinyl chloride) (PVC) | 180–260 °C, 15 h | - | - | [157] | |
| Waste plastic | 250–350 °C, 1 h | - | - | [158] | |
| Polyvinyl chloride–corncob | 260 °C, 60 min | - | 17.80–32.83 MJ/kg | [159] | |
| Polyvinyl chloride (PVC) and pinewood sawdust | 280 °C, 1 h | - | 18.3–30.89 MJ/kg | [160] | |
| PVC-containing medical wastes | 210 °C, 30 min | - | 24.18–30.52 MJ/kg | [161] | |
| Corn stover–polyurethane mixture | 200–260 °C, 30 min | - | 16.8–34.1 MJ/kg | [162] | |
| Poly(vinyl chloride) (PVC) | 180–260 °C | - | - | [157] | |
| Cotton textile waste (CTW) and polyvinyl chloride waste (PVCW) | 240–270 °C, 2 h | HCl | 20.07–30.65 MJ/kg | [163] | |
| Pyrolysis | HDPE, LDPE, IPP, PS, ABS, PE | 420–480 °C | HZSM-5 and PZSM-5 | - | [164] |
| PP, HDPE, LDPE | 500–730 °C | Ziegler-Natta (Z-N): TiCl4/MgCl2 | - | [165] | |
| LDPE and PP | 310–400 °C | Ni/h-ZSM-5, Ni/h-Beta) (Ni/Al-MCM-41, Ni/Al-SBA-15) | - | [166] | |
| Polymer wastes (LDPE/HDPE/PP/PS) | 290–420 °C | USY, MOR, ASA, ZSM-5, MCM-41 | - | [167] | |
| Waste plastics | 430–571 °C | Activated carbon | - | [168] | |
| Mixed plastics and hard-burnt lime | 600–700 °C | CaO, Ca(OH)2, CaCO3 | - | [169] | |
| Mixed plastics | 300–500 °C | AL-PILC | 36.3–42.9 MJ/kg | [170] | |
| ABS/PC and PCB | 500 °C | Y-Zeolite (YZ), ZSM-5, biochar (BC), electronic waste char (EWC), iron oxide loaded YZ (Fe/YZ), Fe/ZSM-5, Fe/BC, Fe/EWC | - | [171] | |
| Waste polyethylene and polypropylene Mixture | 500 °C 600 °C | ZSM-5, AL-MCM-41, AL-SBA-15 H3PO4, activated carbon | - | [172,173] | |
| Municipal plastic waste Plastics wastes (PS, PE, PP, PET) | 700 °C 450 °C | Me/Ni/ZSM-5 Modified natural zeolite | - 41.7–44.2 MJ/kg | [174,175] | |
| Gasification | Waste polyethylene (PE) PE | 700–900 °C 850 °C, 3 h | NiO/ɤ-Al2O3 - | - | [176,177] |
| Polyethylene (PE) and polypropylene (PP) | 900 °C | - | - | [178] | |
| Mixed plastic wastes (mainly polyolefins) | 700 °C, 3 h | Olivine | - | [179] | |
| Recycled plastic (from packaging) | 750 °C, 2 h | - | 18.40–42.69 MJ/kg | [180] | |
| Mixture of waste polyolefins | 700 °C | - | - | [181] | |
| Polypropylene waste | 750–850 °C | Dolomite and olivine | - | [182] | |
| Waste plastic mixture | 800 °C | - | 13.44 MJ/kg | [183,184] | |
| Waste polyolefins | 750 °C | - | - | [185] | |
| Waste plastic mixture Plastic wastes | 500–900 °C, 500–750 °C | - | 10 MJ/(Nm3) | [186,187] | |
| Virgin PE | 350 °C | Ca(OH)2 and Ni(OH) | - | [188] |
| Conversion Process | Feed Stock | Reaction Parameters | Catalyst | HHV | Ref. |
|---|---|---|---|---|---|
| Hydrothermal Liquefaction (HTL) | Lactose, maltose, lysine | 250–350 °C, 20 min | - | - | [197] |
| Montepulciano grape pomace, Cabernet Sauvignon grape pomace, sugar beet tailings, extracted grain | 330–350 °C | - | - | [198] | |
| Tobacco-processing waste | 280–340 °C | - | 26.1–31.9 MJ/kg | [199] | |
| Food waste | 240–295 °C | - | 40.2 MJ/kg | [200] | |
| Food wastes (meat, chesee, fruit) | 300 °C | - | - | [201] | |
| Food wastes | 200–600 °C | - | 35 MJ/kg | [202] | |
| Olive oil waste and hazel nutshell | 180 °C, 4 h | - | - | [203] | |
| Oil palm fruit press fiber | 210–337 °C | ZnCl2, Na2CO3, NaOH | - | [204] | |
| Shrimp shells (seafood-processing waste) | 90–250 °C, 5–60 min | - | - | [205] | |
| Oil mill wastewater (OMWW) | 240–300 °C | - | 38 MJ/kg | [206] | |
| Hydrothermal Carbonization (HTC) | Food waste | 225–275 °C, 24 h | - | - | [207] |
| Crude oil sludge | 150–250 °C | - | - | [208] | |
| Oily sludge | 150–250 °C, 0.5–4 h | - | 18.1–26.7 MJ/kg | [209] | |
| Olive Mill Wastewater | 180–250 °C, 35–55 bar, 2–8 h | - | - | [210] | |
| Agro-industrial waste (Grape marc) | 180–250 °C, 1, 3, 8 h | - | 19.8–24.1 MJ/kg | [211] | |
| Moist agro-industrial waste (olive mill, canned artichoke, orange wastes) | 200–250 °C, 2, 4, 8–24 h | - | 25.09–33.21 MJ/kg | [212] | |
| Apple industry waste | 180–230 °C, 2–4 h | - | 30 MJ/kg | [213] | |
| Pharmaceutical biowastes | 240 °C | - | 24–25 MJ/kg | [214] | |
| Waste textile and waste wood | 240 °C, 90 min | - | 18.04–26.94 MJ/kg | [215] | |
| Antibiotic mycelial dreg | 160–220 °C, 30 min | - | 16.7–24.4 MJ/kg | [216] | |
| Pyrolysis | Olive oil industry waste | 300–500 °C | - | 21.01–31.26 MJ/kg | [26] |
| Olive mill | 400–600 °C | - | 25.74–29.78 MJ/kg | [217] | |
| Industrial wastes (olive and hazelnut bagasse) | 500 °C | Activated alumina and sodium feldspar | 31.80–35.74 MJ/kg | [218] | |
| Heavy shale oil–polyethylene waste | 450 °C | Al-Co-Mo and Akzo Nobel (Mo + S) | - | [219] | |
| Tire industry wastes | 300–600 °C | - | 29.67–37.31 MJ/kg | [220] | |
| Industrial waste (lignin) | 300–700 °C | - | 29.19–31.62 MJ/kg | [221] | |
| Industrial alliaceous plant wastes | 350–700 °C | - | - | [222] | |
| Industrial wastes (herb residue) | 350–550 °C | ZSM-5, Al-SBA-15, alumina | 18.66–25.94 MJ/kg | [223] | |
| Agricultural and industrial wastes | 300–700 °C | - | - | [224] | |
| Industrial waste | 400–700 °C, 300–700 °C, 1–2 h | - | 32–37.91 MJ/kg | [225,226] | |
| Gasification | Palm kernel shell–oil sludge mixture | 400–1000 °C | - | 18.18–42.27 MJ/kg | [227] |
| Baggase residue organic solid wastes, corn stalk (CS), poplar sawdust (PS) | 700–900 °C | - | - | [228] | |
| Olive pomace | 700–900 °C | - | - | [229] | |
| Oily sludge and chicken manure | 700–800 °C | - | - | [230] | |
| Waste tires | 1000 °C | - | - | [231] | |
| Waste tyre | 850–1000 °C | - | - | [232] | |
| Industrial wastewater sludge | 600–800 °C | - | 4.84–5.11 MJ/Nm3 | [233] | |
| Sewage and textile sludge | 850 °C | - | - | [234] | |
| Olive industry wastes | 850 °C | - | 2.83–3.98 MJ/kg | [235] | |
| Municipal sewage sludge | 600–850 °C | - | 4.8–5.4 MJ/kg | [236] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durak, H. Comprehensive Assessment of Thermochemical Processes for Sustainable Waste Management and Resource Recovery. Processes 2023, 11, 2092. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11072092
Durak H. Comprehensive Assessment of Thermochemical Processes for Sustainable Waste Management and Resource Recovery. Processes. 2023; 11(7):2092. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11072092
Chicago/Turabian StyleDurak, Halil. 2023. "Comprehensive Assessment of Thermochemical Processes for Sustainable Waste Management and Resource Recovery" Processes 11, no. 7: 2092. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11072092