1. Introduction
In agricultural areas, modern technologies such as soilless cultured crops, hydroponics and aquaponics are used for vegetable and herb production [
1]. This cropping system has many advantages including the ability to recycle water and nutrients, protect the environment and improve the quality and quantity of the crop [
2].
Lettuce (
Lactuca sativa L.) is one of the most important leafy vegetable crops worldwide. It provides a considerable amount of polyphenolic compounds, vitamins A, C and E, calcium and iron when consumed as fresh green salad [
3]. The continued use of nitrogen fertilizers has increased the nitrate content in vegetable leaves without improving output, raising the risk of agricultural pollution and negatively affecting human health [
4]. However, to overcome these problems, there is an alternative method of agricultural management, which is often referred to as a challenge for sustainability in agriculture, and ecologically sound means to reduce the use of inorganic fertilizers by organizing programs to increase agricultural productivity, especially in vegetable production. Therefore, clean techniques such as biostimulants are considered an alternative source of nutrition for production [
1,
5].
Previous research has extensively investigated the use of plant growth promoting rhizobacteria (PGPR) as biofertilizers to promote plant growth [
6,
7,
8,
9]. Foliar and root application of PGPR has been shown to increase macro- and micronutrient content, induce systemic resistance to pathogens and withstand environmental stress conditions [
9,
10,
11].
In addition, microorganisms such as Pseudomonas,
Azotobacter and
Azospirillum can produce different types of plant hormones [
2,
11,
12]. In the PGPR group,
Azotobacter, which belongs to the Gammaproteobacteria group, can fix atmospheric nitrogen and grow effectively under nitrogen-free conditions. They synthesize cellular proteins by utilizing atmospheric nitrogen. The availability of nitrogen is related to cell death after mineralization of the cellular protein [
13]. Moreover,
Azotobacter strains showed positive effects on plant growth, crop yield and nitrogen requirement of horticultural crops and achieved significant yield increases (up to 40%) [
6,
11,
14]. Ahmed et al. [
12] found that treatment of lettuce plants with
Azotobacter chroococcum and
Azospirillum lipoferum as biofertilizers resulted in significant increases in aerial plant height, leaf number and fresh weight. A significant decrease in nitrate accumulation was observed when the plants were treated with biofertilizer. Foliar application of nitrogen fixing bacteria, namely
Azotobacter,
Azospirillum and
Beijerinckia, resulted in improved leaf quality as specified by their protein content and effect on breeding in mulberry plants [
6].
Azotobacter spp. and
Azospirillum spp. showed significant increases in chlorophyll content in hydroponically grown strawberries under different nitrogen concentrations [
11].
PGPR are highly effective in promoting plant growth. However, information on the effects of foliar application of PGPR on plant growth, physicochemical properties and nitrate accumulation in greenhouse lettuce in a soilless crop with different nitrogen levels is lacking and inconclusive. Therefore, this study aimed to investigate the effects of foliar spraying of Azotobacter on growth parameters, antioxidant enzyme activities and macronutrient content of hydroponically grown lettuce with different levels of nitrogen in nutrient solution. This is the first report on the effects of foliar application of PGPR on lettuce cultivation.
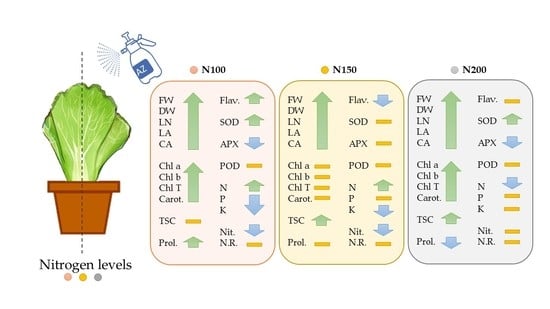
3. Discussion
Nitrogen (N), one of the most important macronutrients, significantly determines crop yield and quality [
15]. Moreover, several species have been reported to have enhanced growth and development in plants fertilized with different nitrogen concentrations or inoculated with PGPR in hydroponics [
11,
16,
17,
18,
19]. The data obtained showed that the effects of different nitrogen concentrations on lettuce sprayed with PGPR were consistent with previous reports, with plant growth showing an increasing trend [
11,
20].
Azotobacter sprayed lettuce increased vegetative growth expressed in number of leaves, fresh and dry weight of head, leaf and canopy area. Similar results were reported by Al-Taey and Majid (2018) [
21] for lettuce and by Al-Taey et al. [
1] for broccoli. Sudhakar et al. [
6] found that foliar application of biofertilizers, especially
Azotobacter, significantly improved the growth parameters of mulberry plants compared to the untreated plants. The positive effect of
Azotobacter on plant growth can be attributed to the fixation of sufficient nitrogen on the leaf surface, production of phytohormones such as auxins, gibberellins and cytokinins that alter plant growth and morphology, and its antagonistic effect on fungi and plant pathogenic bacteria [
6,
11,
14,
17,
18,
22]. Accordingly, this is the first study on the use of nitrogen-fixing bacteria by foliar spraying in lettuce under soilless culture. Foliar application of
Azotobacter improved the availability of leaf nutrient content and some growth-promoting substances, which contributed to an increase in leaf number, leaf area, fresh weight and dry weight of head, and canopy area of soilless grown lettuce. These desirable conditions resulted in higher photosynthetic activity by utilizing more light energy and CO
2, which in turn increased the synthesis of metabolic materials and translocation, accumulation of dry matter and its content in the lettuce leaves. Similarly, growth and productivity of most vegetables are related to adequate nitrogen supply in nutrient solutions [
23], which is in agreement with the current study. Tei et al., (2000) suggested that nitrogen addition in nutrient solution leads to improvement in vegetative growth and is responsible for improved leaf area and increased chlorophyll concentration as a result of impressive utilization of photosynthetically active radiation [
24]. While in the present study, 200 mg/L nitrogen resulted in positive effect on vegetative growth of lettuce plant, Becker et al., (2015) found that plant growth was affected by low nitrogen concentrations [
25]. Leaf and canopy area increased even further when the combination of
Azotobacter with nitrogen fertilizer was used, which was in agreement with the study of Umar et al. [
26]. The results of this study indicated that nitrogen addition in the solution and growth stimulating bacteria increased photosynthetic pigments in soilless grown lettuce. Moreover, many authors have confirmed these results [
27,
28,
29,
30]. Since chlorophyll is an important index for evaluating the growth and development of lettuce plants, the application of nitrogen (a key factor for chlorophyll formation) and
Azotobacter appeared to promote the development of chlorophyll, its synthesis, the induction of chlorophyll-related enzymes and the activity of photosynthetic enzymes, resulting in the regulation of photosynthesis [
27,
29,
30].
The increase in carotenoid content with the application of
Azotobacter and nitrogen sources has elucidated the key role of these compounds in the photosynthetic process of lettuce growth, photosystem support, induction of carotenoid protection and prevention of carotenoid oxidation by reactive oxygen species [
28,
29,
31].
Foliar application of growth-stimulating bacteria and different levels of nitrogen in hydroponically grown lettuce did not increase protein content, which was not consistent with the results of Pan et al., [
25] and Latef et al. [
28,
32].
Increased nitrogen applications in the nutrient solution of soilless grown lettuce can increases proline accumulation in lettuce leaves [
30]. The reports of Sánchez et al. also confirmed that the accumulation of this nitrogenous form can act as osmolyte and other important cellular functions [
33]. Accordingly, foliar spraying of
Azotobacter reduced proline content in lettuce leaves when treated with 200 mg/L nitrogen. It appeared that the application of bacteria led to a reduction of this nitrogen excess by improving the availability of leaf nutrient content and some growth promoters. The higher nitrogen content in combination with
Azotobacter resulted in a significant increase in soluble sugar in the lettuce leaves. This increase in total soluble sugar is due to the enhancement of the photosynthetic system of the host plants, which is in agreement with the study of Arough et al. [
13] in triticale.
Secondary metabolites such as phenols and flavonoids are considered antioxidants that protect against oxidative damage and play an important role in human nutrition [
16,
20]. In agreement with previous findings [
34], the present results again showed that antioxidant activity and total phenolic content, the most important quality variables in lettuce plants, were not affected by nitrogen content. Nevertheless, the changes in total phenolic content and antioxidant activity are associated with increased nitrogen applications as reported by Mampholo et al. [
16]. Flavonoid content showed a semi-downward trend with increasing nitrogen application rates in lettuce plants. This confirms earlier findings of Qadir et al. [
32], who concluded that increasing nitrogen application rates led to a decrease in flavonoid content of lettuce [
35].
While reactive oxygen species are vital in host defense, ROS residues can induce oxidative damage [
36]. SOD, POD, APX and CAT are antioxidant enzymes that are critical in detecting and detoxifying excess reactive oxygen species, and their activities may be elevated following exposure to moderate environmental stresses [
36,
37,
38]. In our investigation, the activities of SOD and POD rose significantly as nitrogen (N) levels increased. SOD activity, on the other hand, increased with increasing N concentration up to a specific level (150 mg/L). According to Liao et al. [
39], the activity of these enzymes may rise at low levels of N supplementation but may be suppressed at high levels. Changes in SOD activity caused by
Azotobacter treatment may potentially be due to altered synthesis and accumulation of less active enzymes, as well as higher SOD turnover [
40,
41]. The increase in antioxidant enzyme activity did not relieve ROS toxicity, resulting in photosynthesis damage and chlorophyll degradation. According to Wang et al. [
42], appropriate N dosage can improve the activity of antioxidant enzymes, increase the scavenging ability of mesophyll cells against ROS and maintain cell integrity, all of which increase yield [
42].
Several studies indicate that the physical and chemical properties of the growth substrate and inoculation with biofertilizers can influence the mineral composition of plants by increasing the content of nutrients in terms of health benefits for humans [
20]. The current study is in line with previous studies which showed that nitrogen content in leaves increases in line with nitrogen supply in the nutrient solution [
43].
It is interesting to note that P and K content peaked at the lowest nitrogen additions in hydroponically grown lettuce. It seems that the increase in leaf area along with the dilution of minerals as the nitrogen content of the solution increases contributes to ion antagonism and decrease in mineral concentration [
15].
Nitrate accumulation in vegetables is influenced by several factors, including nitrogen amounts and sources, light intensity and temperature and internal factors such as genotype and species [
29,
44]. In addition, the activity of the enzyme nitrate reductase is influenced by the nitrate availability of leaves under soilless culture. The use of lower nitrogen content in the nutrient solution resulted in a decrease in nitrate content in lettuce leaves, as previously shown by Balanz et al. [
45]. The application of
Azotobacter resulted in a decrease in nitrate content in the leaves compared to the control, possibly as a result of the utilization of nitrates by the bacteria in an anaerobic respiration process [
45]. The results showed that the higher nitrate concentrations in the solution did not result in higher enzyme activity. This observation differs from the reports of Wenceslau et al. [
19]. It seems that not only nitrate availability affects nitrate reductase activity, but also other factors such as environmental conditions should be considered. Lower light intensity may lead to greater accumulation of nitrate in leafy vegetables by decreasing nitrate reductase activity, possibly a similar mechanism followed in the current study [
44].