The Control of the Expansion or Compression of Colloidal Crystals Lattice with Salt Solution
Abstract
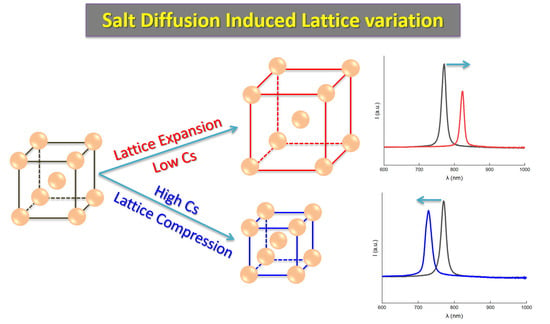
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Experimental Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Yu, S.; Wei, B.; Yang, D.; Ma, D.; Huang, S. Stimulus-responsive nonclose-packed photonic crystals: Fabrications and applications. Mater. Horiz. 2023, 10, 3895–3928. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, Z.W.; Ravaine, S.; He, M.X.; Song, Y.L.; Yin, Y.D.; Zheng, H.B.; Teng, J.H.; Zhang, A.O. From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Yin, Y.D. Stimuli-Responsive Optical Nanomaterials. Adv. Mater. 2019, 31, 1807061. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, M.; Song, Y. Recent advances in colloidal photonic crystal sensors: Materials, structures and analysis methods. Nano Today 2018, 22, 132–144. [Google Scholar] [CrossRef]
- Neterebskaia, V.O.; Goncharenko, A.O.; Morozova, S.M.; Kolchanov, D.S.; Vinogradov, A.V. Inkjet Printing Humidity Sensing Pattern Based on Self-Organizing Polystyrene Spheres. Nanomaterials 2020, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Yu, W.; Fu, Q.; Ge, J. Low-Voltage Electrically Responsive Photonic Crystal Based on Weak-Polar Colloidal System. Adv. Opt. Mater. 2022, 10, 2201188. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, H.; Ge, J. Electrically Tunable Liquid Photonic Crystals with Large Dielectric Contrast and Highly Saturated Structural Colors. Adv. Funct. Mater. 2018, 28, 1804628. [Google Scholar] [CrossRef]
- Xu, X.; Friedman, G.; Humfeld, K.D.; Majetich, S.A.; Asher, S.A. Synthesis and Utilization of Monodisperse Superparamagnetic Colloidal Particles for Magnetically Controllable Photonic Crystals. Chem. Mater. 2002, 14, 1249–1256. [Google Scholar] [CrossRef]
- Ge, J.P.; He, L.; Goebl, J.; Yin, Y.D. Assembly of Magnetically Tunable Photonic Crystals in Nonpolar Solvents. J. Am. Chem. Soc. 2009, 131, 3484–3486. [Google Scholar] [CrossRef]
- Lee, G.H.; Choi, T.M.; Kim, B.; Han, S.H.; Lee, J.M.; Kim, S.H. Chameleon-Inspired Mechanochromic Photonic Films Composed of Non-Close-Packed Colloidal Arrays. ACS Nano 2017, 11, 11350–11357. [Google Scholar] [CrossRef]
- Yang, D.; Ye, S.; Ge, J. From Metastable Colloidal Crystalline Arrays to Fast Responsive Mechanochromic Photonic Gels: An Organic Gel for Deformation-Based Display Panels. Adv. Funct. Mater. 2014, 24, 3197–3205. [Google Scholar] [CrossRef]
- Chan, E.P.; Walish, J.J.; Thomas, E.L.; Stafford, C.M. Block copolymer photonic gel for mechanochromic sensing. Adv. Mater. 2011, 23, 4702–4706. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.A.; Holtz, J.; Liu, L.; Wu, Z.J. Self-assembly Motif for Creating Submicron Periodic Materials—Polymerized Crystalline Colloidal Arrays. J. Am. Chem. Soc. 1994, 116, 4997–4998. [Google Scholar] [CrossRef]
- Yamanaka, J.; Murai, M.; Iwayama, Y.; Yonese, M.; Ito, K.; Sawada, T. One-Directional Crystal Growth in Charged Colloidal Silica Dispersions Driven by Diffusion of Base. J. Am. Chem. Soc. 2004, 126, 7156–7157. [Google Scholar] [CrossRef] [PubMed]
- Toyotama, A.; Yamamoto, M.; Nakamura, Y.; Yamazaki, C.; Tobinaga, A.; Ohashi, Y.; Okuzono, T.; Ozaki, H.; Uchida, F.; Yamanaka, J. Thermoresponsive Colloidal Crystallization Using Adsorption of Ionic Surfactants. Chem. Mater. 2014, 26, 4057–4059. [Google Scholar] [CrossRef]
- Yamanaka, J.; Okuzono, T.; Toyotama, A. Fundamentals of Colloidal Self-Assembly. In Colloidal Self-Assembly; Yamanaka, J., Okuzono, T., Toyotama, A., Eds.; Springer Nature: Singapore, 2023; pp. 13–40. [Google Scholar]
- Spada, E.R.; de Paula, F.R.; Pla Cid, C.C.; Candiotto, G.; Faria, R.M.; Sartorelli, M.L. Role of acidic and basic electrolytes on the structure and morphology of cathodically reduced indium tin oxide (ITO) substrates. Electrochim. Acta 2013, 108, 520–524. [Google Scholar] [CrossRef]
- Shim, H.; Shin, C.G.; Heo, C.-J.; Jeon, S.-J.; Jin, H.; Kim, J.W.; Jin, Y.; Lee, S.; Lim, J.; Han, M.G.; et al. Stability enhancement of an electrically tunable colloidal photonic crystal using modified electrodes with a large electrochemical potential window. Appl. Phys. Lett. 2014, 104, 051104. [Google Scholar] [CrossRef]
- Macher, S.; Rumpel, M.; Schott, M.; Posset, U.; Giffin, G.A.; Lobmann, P. Avoiding Voltage-Induced Degradation in PET-ITO-Based Flexible Electrochromic Devices. ACS Appl. Mater. Interfaces 2020, 12, 36695–36705. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Dukhin, S.S.; Korotkova, A.A. Diffusiophoresis in electrolyte solutions and its role in the Mechanism of the formation of films from caoutchouc latexes by the ionic deposition method. Prog. Surf. Sci. 1993, 43, 153–158. [Google Scholar] [CrossRef]
- Samanta, S.; Mahapatra, P.; Ohshima, H.; Gopmandal, P.P. Diffusiophoresis of hydrophobic spherical particles in a solution of general electrolyte. Phys. Fluids 2023, 35, 032006. [Google Scholar] [CrossRef]
- Shim, S. Diffusiophoresis, Diffusioosmosis, and Microfluidics: Surface-Flow-Driven Phenomena in the Presence of Flow. Chem. Rev. 2022, 122, 6986–7009. [Google Scholar] [CrossRef]
- Shin, S. Diffusiophoretic separation of colloids in microfluidic flows. Phys. Fluids 2020, 32, 101302. [Google Scholar] [CrossRef]
- Velegol, D.; Garg, A.; Guha, R.; Kar, A.; Kumar, M. Origins of concentration gradients for diffusiophoresis. Soft Matter 2016, 12, 4686–4703. [Google Scholar] [CrossRef]
- Saad, S.; Natale, G. Diffusiophoresis of active colloids in viscoelastic media. Soft Matter 2019, 15, 9909–9919. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Choudhury, U.; Fischer, P.; Mark, A.G. Non-Equilibrium Assembly of Light-Activated Colloidal Mixtures. Adv. Mater. 2017, 29, 1701328. [Google Scholar] [CrossRef] [PubMed]
- Bekir, M.; Sharma, A.; Umlandt, M.; Lomadze, N.; Santer, S. How to Make a Surface Act as a Micropump. Adv. Mater. Interfaces 2022, 9, 2102395. [Google Scholar] [CrossRef]
- Shimokusu, T.J.; Maybruck, V.G.; Ault, J.T.; Shin, S. Colloid Separation by CO2-Induced Diffusiophoresis. Langmuir 2020, 36, 7032–7038. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Park, S.; Lee, D.; Lee, H.; Kim, S.J. Continuous and spontaneous nanoparticle separation by diffusiophoresis. Lab Chip 2020, 20, 4118–4127. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Botin, D.; Weber, J.; Reinmuller, A.; Palberg, T. Assembly and Speed in Ion-Exchange-Based Modular Phoretic Microswimmers. Langmuir 2017, 33, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Kreissl, P.; Brown, A.T.; Rempfer, G.; Botin, D.; Holm, C.; Palberg, T.; de Graaf, J. Microfluidic pumping by micromolar salt concentrations. Soft Matter 2017, 13, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Gustafsson, J.; Mikkola, P.; Jokinen, M.; Rosenholm, J.B. The influence of pH and NaCl on the zeta potential and rheology of anatase dispersions. Colloids Surf. A 2000, 175, 349–359. [Google Scholar] [CrossRef]
- Nattich-Rak, M.; Sadowska, M.; Adamczyk, Z.; Ciesla, M.; Kakol, M. Formation mechanism of human serum albumin monolayers on positively charged polymer microparticles. Colloids Surf. B 2017, 159, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E.; Dhinojwala, A.; Moore, F.B. Effect of Substrate and Bacterial Zeta Potential on Adhesion of Mycobacterium smegmatis. Langmuir 2019, 35, 7035–7042. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.S.; Saingam, P.; Yan, T.; Shin, S. A Trace Amount of Surfactants Enables Diffusiophoretic Swimming of Bacteria. ACS Nano 2020, 14, 14219–14227. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.F.; Islam, M.A.; Khorshidi, B.; Sadrzadeh, M. Prediction of surface charge properties on the basis of contact angle titration models. Mater. Chem. Phys. 2021, 258, 123933. [Google Scholar] [CrossRef]
- Sureda, M.; Miller, A.; Diez, F.J. In situ particle zeta potential evaluation in electroosmotic flows from time-resolved microPIV measurements. Electrophoresis 2012, 33, 2759. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, B.; Wood, J.A.; Lammertink, R.G.H. Diffusiophoresis and Diffusio-osmosis into a Dead-End Channel: Role of the Concentration-Dependence of Zeta Potential. Langmuir 2023, 39, 2322–2332. [Google Scholar] [CrossRef]
- Zhou, H.W.; Xu, S.H.; Sun, Z.W.; Du, X.; Xie, J.C. Rapid determination of colloidal crystal’s structure by reflection spectrum. Colloids Surf. A 2011, 375, 50–54. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; Zhao, X.; Xu, S. Experimental observation of effect of the wall curvature of capillary tube on colloidal crystallization inside the tube. Colloids Surf. A 2020, 603, 125294. [Google Scholar] [CrossRef]
- Shim, T.S.; Kim, S.H.; Sim, J.Y.; Lim, J.M.; Yang, S.M. Dynamic modulation of photonic bandgaps in crystalline colloidal arrays under electric field. Adv. Mater. 2010, 22, 4494. [Google Scholar] [CrossRef]
- Riquet, A.M.; Rohman, G.; Guinault, A.; Demilly, M. Surface modification of polypropylene by radiation grafting of hydrophilic monomers: Physicochemical properties. Surf. Eng. 2013, 27, 234–241. [Google Scholar] [CrossRef]
- Frank, J.; Simon, F.; Schmitt, F.J. Characterization of the interfacial properties of modified polypropylene. Phys. Chem. Chem. Phys. 1999, 1, 3865–3869. [Google Scholar] [CrossRef]
- Namie, M.; Kim, J.-H.; Yonezawa, S. Improving the Dyeing of Polypropylene by Surface Fluorination. Colorants 2022, 1, 121–131. [Google Scholar] [CrossRef]
- Lukowiak, M.C.; Wettmarshausen, S.; Hidde, G.; Landsberger, P.; Boenke, V.; Rodenacker, K.; Braun, U.; Friedrich, J.F.; Gorbushina, A.A.; Haag, R. Polyglycerol coated polypropylene surfaces for protein and bacteria resistance. Polym. Chem. 2015, 6, 1350–1359. [Google Scholar] [CrossRef]







| Cs (M) | D (mm) | (nm) | (nm) | |
|---|---|---|---|---|
| 2 × 10−4 | 4.4 | 54 | 29 | 0.07 |
| 6.9 | 41 | 22 | 0.05 | |
| 8.6 | 28 | 15 | 0.04 | |
| 10.5 | 19 | 10 | 0.02 | |
| 5 × 10−3 | 2.6 | −43 | −23 | −0.06 |
| 6.0 | −33 | −17 | −0.04 | |
| 9.0 | −31 | −16 | −0.04 | |
| 13.2 | −27 | −14 | −0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Ouyang, W.; Zou, S.; Xu, S. The Control of the Expansion or Compression of Colloidal Crystals Lattice with Salt Solution. Nanomaterials 2024, 14, 355. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14040355
Zhou H, Ouyang W, Zou S, Xu S. The Control of the Expansion or Compression of Colloidal Crystals Lattice with Salt Solution. Nanomaterials. 2024; 14(4):355. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14040355
Chicago/Turabian StyleZhou, Hongwei, Wenze Ouyang, Shuangyang Zou, and Shenghua Xu. 2024. "The Control of the Expansion or Compression of Colloidal Crystals Lattice with Salt Solution" Nanomaterials 14, no. 4: 355. https://0-doi-org.brum.beds.ac.uk/10.3390/nano14040355