Intense Blue Photo Emissive Carbon Dots Prepared through Pyrolytic Processing of Ligno-Cellulosic Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Preparation of the CDs Derived from Forestry Lingo-Cellulosic Waste
3. Results
3.1. EDX Investigation
3.2. Thermal Analysis
3.3. FT-IR Analysis
3.4. Raman Spectroscopy
3.5. XRD Analysis
3.6. Dimensional Analysis DLS
3.7. HR-SEM Imaging
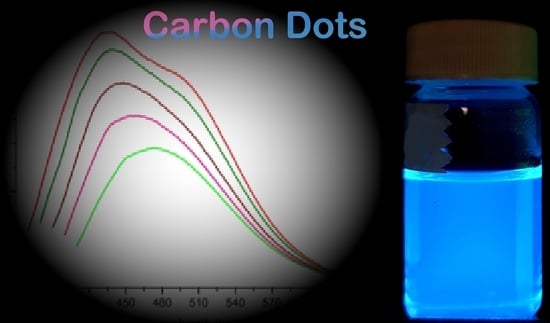
3.8. Fluorescence Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuerhong, M.; Xu, Y.; Yin, X.B. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Jorns, M.; Pappas, D. A Review of Fluorescent Carbon Dots, Their Synthesis, Physical and Chemical Characteristics, and Applications. Nanomaterials 2021, 11, 1448–1466. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Yang, B. Bioimaging based on fluorescent carbon dots. RSC Adv. 2014, 4, 27184–27200. [Google Scholar] [CrossRef]
- Tan, D.; Zhou, S.; Shimotsuma, Y.; Miura, K.; Qiu, J. Effect of UV irradiation on photoluminescence of carbon dots. Opt. Mater. Express 2014, 4, 213–219. [Google Scholar] [CrossRef]
- Siddique, A.B.; Hossain, S.M.; Pramanick, A.K.; Ray, M. Excitation dependence and independence of photoluminescence in carbon dots and graphene quantum dots: Insights into the mechanism of emission. Nanoscale 2021, 13, 16662–16671. [Google Scholar] [CrossRef]
- Hola, K.; Bourlinos, A.B.; Kozak, O.; Berka, K.; Siskova, K.M.; Havrdova, M.; Tucek, J.; Safarova, K.; Otyepka, M.; Giannelis, E.P.; et al. Photoluminescence effects of graphitic core size and surface functional groups in carbon dots: COO− induced red-shift emission. Carbon 2014, 70, 279–286. [Google Scholar] [CrossRef]
- Jiang, Z.; Krysmann, M.J.; Kelarakis, A.; Koutnik, P.; Anzenbacher, P., Jr.; Roland, P.J.; Ellingson, R.; Sun, L. Understanding the Photoluminescence Mechanism of Carbon Dots. MRS Adv. 2017, 2, 2927–2934. [Google Scholar] [CrossRef] [Green Version]
- Zulfajri, M.; Sudewi, S.; Ismulyati, S.; Rasool, A.; Adlim, M.; Huang, G.G. Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review. Coating 2021, 11, 1100. [Google Scholar] [CrossRef]
- Gou, H.; Liu, Y.; Zhang, G.; Liao, Q.; Huang, X.; Ning, F.; Ke, C.; Meng, Z.; Xi, K. Lifetime-tunable room-temperature phosphorescence of polyaniline carbon dots in adjustable polymer matrices. Nanoscale 2019, 11, 18311–18319. [Google Scholar] [CrossRef]
- Colusso, E.; Cicerchia, L.; Rigon, M.; Gomes, V.; Martucci, A. Photoluminescence properties of silk–carbon quantum dots composites. J. Sol.-Gel. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Chaudhary, R.; Varadarajan, V.; Mohanty, S.K.; Koymen, A.R. Fluorescent carbon nanoparticles synthesized from benzene by electric plasma discharge. Nanoscale 2011, 79080C, 71–79. [Google Scholar]
- Thongpool, V.; Asanithi, P.; Limsuwan, P. Synthesis of Carbon Particles using Laser Ablation in Ethanol. Procedia Eng. 2012, 32, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.W.; Romainor, A.N.B.; Chin, S.F.; Ng, S.M. Carbon dots production via pyrolysis of sago waste as potential probe for metal ions sensing. J. Anal. Appl. Pyrol. 2014, 105, 157–165. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Sonker, A.K.; Sonkar, S.K.; Sarkar, S. Pollutant soot of diesel engine exhaust transformed to carbon dots for multicoloured imaging of E. coli and sensing cholesterol. RSC Adv. 2014, 4, 30100–30107. [Google Scholar] [CrossRef]
- Papaioannou, N.; Marinovic, A.; Yoshizawa, N.; Goode, A.E.; Fay, M.; Khlobystov, A.; Titirici, M.M.; Sapelkin, A. Structure and solvents effects on the optical properties of sugar-derived carbon nanodots. Nature Sci. Rep. 2018, 8, 6559. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic Safety Evaluation on Photoluminescent Carbon Dots. Nanoscale Res. Lett. 2013, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yang, X.; Zheng, J.; Li, Y.; Huang, C. Ultrasonic one-step synthesis of biocompatible yellow-green fluorescent carbon dots. Sci. Sin. Chim. 2013, 43, 895–900. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Wang, D.; Li, Z.; Primo, F.L.; Tedesco, A.C.; Bi, H. Copper-Doped Carbon Dots for Optical Bioimaging and Photodynamic Therapy. Inorg. Chem. 2019, 58, 13394–13402. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Q.; Xiong, Y.; Ji, T.; Wang, C.; Shen, X.; Lu, M.; Wang, H.; Wen, S.; Zhang, Y.; et al. Energy Level Modification with Carbon Dot Interlayers Enables Efficient Perovskite Solar Cells and Quantum Dot Based Light-Emitting Diodes. Adv. Funct. Mater. 2020, 30, 1910530. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef]
- Yao, B.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots: A small conundrum. Trends Chem. 2019, 1, 235–246. [Google Scholar] [CrossRef]
- Tan, M.; Zhang, L.; Tang, R.; Song, X.; Li, Y.; Wu, H.; Wang, Y.; Lv, G.; Liu, W.; Ma, X. Enhanced photoluminescence and characterization of multicolor carbon dots using plant soot as a carbon source. Talanta 2013, 115, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, H.; Song, X.; Ma, X.; Wang, J.; Tan, M. Presence of photoluminescent carbon dots in Nescafe® original instant coffee: Applications to bioimaging. Talanta 2014, 127, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.A.H.; Harrison, J.; Rooney, D.W. The production and application of carbon nanomaterials from high alkali silicate herbaceous biomass. Sci. Rep. 2020, 10, 2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Piao, G.; Zhang, J.; Bu, C.; Xie, H.; Wu, B.; Kobayashi, N. Synthesis of carbon nanotubes from conventional biomass-based gasification gas. Fuel Process. Technol. 2018, 180, 105–113. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Liu, W.; Yang, L.; Zhang, B.; Wang, L.P.; Ji, G.; Xu, Z.J. Biomass-Derived Porous Carbon-Based Nanostructures for Microwave Absorption. Nano-Micro Lett. 2019, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Liu, Y.; Ju, Z.; Luo, J.; Sheng, O.; Nai, J.; Liu, T.; Zhou, Y.; Wang, Y.; Tao, X. Synthesis of Diverse Green Carbon Nanomaterials through Fully Utilizing Biomass Carbon Source Assisted by KOH. ACS Appl. Mater. Interfaces 2019, 11, 24205–24211. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, S.; Zhang, Q.; Chmely, S.C.; Wu, Y.; Melcher, C.; Rajan, K.; Harper, D.P.; Wang, S.; Chen, Z. Recycling hot-water extractions of lignocellulosic biomass in bio-refinery for synthesis of carbon nanoparticles with amplified luminescence and its application in temperature sensing. Ind. Crops Prod. 2020, 145, 112066. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Functionalized Activated Carbon Derived from Biomass for Photocatalysis Applications Perspective. Int. J. Photoenergy 2015, 3, 218743. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Lu, S.; Yang, B. Carbon-Dot-Enhanced Electrocatalytic Hydrogen Evolution. Acc. Mater. Res. 2022, 3, 319–330. [Google Scholar]
- Stan, C.S.; Coroabă, A.; Ursu, E.L.; Secula, M.S.; Simionescu, B.C. Fe(III) doped carbon nanodots with intense green photoluminescence and dispersion medium dependent emission. Nat. Sci. Rep. 2019, 9, 18893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bożym, M.; Gendek, A.; Siemiątkowski, G.; Aniszewska, M.; Malaťák, J. Assessment of the Composition of Forest Waste in Terms of Its Further Use. Materials 2021, 14, 973. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, P. Nickel in forests—A short review on its distribution and fluxes. Folia Oecol. 2021, 48, 205–214. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose Fibers Hydrophobization via a Hybrid Chemical Modification. Polymer 2019, 11, 1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horikawa, Y.; Hirano, S.; Mihashi, A.; Kobayashi, Y.; Zhai, S.; Sugiyama, J. Prediction of Lignin Contents from Infrared Spectroscopy: Chemical Digestion and Lignin/Biomass Ratios of Cryptomeria japonica. Appl. Biochem. Biotechnol. 2019, 188, 1066–1076. [Google Scholar] [CrossRef]
- Rashid, T.; Kait, C.F.; Murugesan, T. “Fourier Transformed Infrared” Compound Study of Lignin Recovered from a Formic Acid Process. Procedia Eng. 2016, 148, 1312–1319. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.; Zhang, P.; Liu, C.; Bai, T.; Li, W.; Dai, L.; Liu, W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 2012, 48, 7955–7957. [Google Scholar] [CrossRef]
- Wu, X.; Tian, F.; Wang, W.; Chen, J.; Wu, M.; Zhao, J.X. Fabrication of highly fluorescent graphene quantum dots using L-glutamic acid for in vitro/in vivo imaging and sensing. J. Mater. Chem. 2013, 1, 4676–4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorio, A.; Ferreira, E.H.M.; Moutinho, M.V.; Stavale, F.; Achete, C.A.; Capaz, R.B. Measuring disorder in graphene with the G and D bands. Phys. Status Solidi B 2010, 247, 2980–2982. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Nagaraju, G.; Nagabhushana, H. Biocompatible synthesis of reduced graphene oxide from Euphorbia heterophylla (L.) and their in-vitro cytotoxicity against human cancer cell lines. Biotechnol. Rep. 2019, 24, e00376. [Google Scholar]
- Srivastava, I.; Khamo, J.S.; Pandit, S.; Fathi, P.; Huang, X.; Cao, A.; Haasch, R.T.; Nie, S.; Zhang, K.; Pan, D. Influence of Electron Acceptor and Electron Donor on the Photophysical Properties of Carbon Dots: A Comparative Investigation at the Bulk-State and Single-Particle Level. Adv. Funct. Mate. 2019, 29, 1902466. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, X.; Wang, F.; Zhang, X.; Zhao, D.; Shen, D. Environment-dependent photon emission from solid state carbon dots and its mechanism. Nanoscale 2014, 17, 10388–10393. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Fu, M.; Ehrat, F.; Wang, Y.; Milowska, K.Z.; Reckmeier, C.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Carbon dots: A unique fluorescent cocktail of polycyclic aromatic hydrocarbons. Nano Lett. 2015, 15, 6030–6035. [Google Scholar] [CrossRef]
- Barman, M.K.; Patra, A. Current status and prospects on chemical structure driven photoluminescence behaviour of carbon dots. J. Photochem. Photobiol. 2018, 37, 1–22. [Google Scholar] [CrossRef]









| Element | Weight % | Atomic % |
|---|---|---|
| C K | 62.8 | 68.7 |
| NK | 6.8 | 6.4 |
| O K | 30.2 | 24.7 |
| FeK | 0.1 | 0.1 |
| NiK | 0.1 | 0.1 |
| Wavenumber (cm−1) | Assignement |
|---|---|
| 3405 | −OH stretching |
| 2925 | C−H stretching |
| 1727 | C=O stretching in acetyl and carboxyl groups |
| 1613 | C=O stretching in carbonyl groups |
| 1516 | Specific vibration of lignin aromatic ring |
| 1442 | O−H in plane bending, C-H bending |
| 1516 | Specific vibration of lignin aromatic ring |
| 1442 | O−H in plane bending, C-H bending |
| 1370 | C−H bending (cellulose, hemicellulose) |
| 1317 | O−H in plane bending (cellulose, hemicellulose) |
| 1275 | C-O stretching (lignin) |
| 1157 | C1−O−C4 anti-symetric bending (cellulose, hemicellulose) |
| 1064 | C6−O6H stretching (cellulose), C−O stretching (lignin) |
| 897 | C=C, C−H bending |
| 771 | C−H bending |
| 528 | C−OH out of plane bending |
| Excitation (nm) | Emission Peak (nm) |
|---|---|
| 350 | 438 |
| 360 | 440 |
| 370 | 448 |
| 380 | 458 |
| 390 | 473 |
| Lifetimes (ns) | Contribution (%) |
|---|---|
| τ1 = 2.585 | 46.86 |
| τ2 = 0.501 | 20.90 |
| τ3 = 8.337 | 32.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stan, L.; Volf, I.; Stan, C.S.; Albu, C.; Coroaba, A.; Ursu, L.E.; Popa, M. Intense Blue Photo Emissive Carbon Dots Prepared through Pyrolytic Processing of Ligno-Cellulosic Wastes. Nanomaterials 2023, 13, 131. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13010131
Stan L, Volf I, Stan CS, Albu C, Coroaba A, Ursu LE, Popa M. Intense Blue Photo Emissive Carbon Dots Prepared through Pyrolytic Processing of Ligno-Cellulosic Wastes. Nanomaterials. 2023; 13(1):131. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13010131
Chicago/Turabian StyleStan, Loredana, Irina Volf, Corneliu S. Stan, Cristina Albu, Adina Coroaba, Laura E. Ursu, and Marcel Popa. 2023. "Intense Blue Photo Emissive Carbon Dots Prepared through Pyrolytic Processing of Ligno-Cellulosic Wastes" Nanomaterials 13, no. 1: 131. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13010131