Thermal Traits of MNPs under High-Frequency Magnetic Fields: Disentangling the Effect of Size and Coating
Abstract
:1. Introduction
2. Experimental Methods
2.1. Synthesis of MNPs
2.2. Characterization of MNPs
2.3. Heating Properties of MNPs
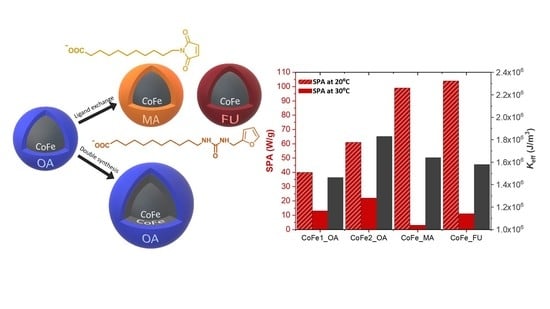
3. Results and Discussion
3.1. Basic Structural and Magnetic Properties
3.2. Heating Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MNP | Magnetic Nanoparticle |
| NP | Nanoparticle |
| SPA | Specific Power Absorption |
| MFH | Magnetic Fluid Hyperthermia |
| OA | Oleic Acid |
| MF | Magnetic Field |
| MA | 11-Maleimidoundecanoic Acid |
| FU | 11-(Furfurylureido)undecanoic Acid |
| TGA | Thermogravimetric Analysis |
| HR TEM | High-Resolution Transmission Electron Microscope |
| DLS | Dynamic Light Scattering |
| DRIFTS | Diffuse Reflectanceinfrared Fourier Transform Spectroscopy |
| SQUID | Superconducting Quantum Interference Device |
References
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the Application of Magnetic Nanoparticles for Sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Hasan, M.; Mathewson, A.; Razeeb, K.M. Disposable sensor based on enzyme-free Ni nanowire array electrode to detect glutamate. Biosens. Bioelectron. 2013, 40, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Horvat, J.; Kim, H.S.; Kim, W.S.; Ahn, J.; Wang, G. Synthesis of Mesoporous α-Fe2O3 Nanostructures for Highly Sensitive Gas Sensors and High Capacity Anode Materials in Lithium Ion Batteries. J. Phys. Chem. C 2010, 114, 18753–18761. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Gao, W.; Wang, J. Synthetic micro/nanomotors in drug delivery. Nanoscale 2014, 6, 10486–10494. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Inorganic Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, I.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamiya, H.; Jeyadevan, B. Hyperthermic effects of dissipative structures of magnetic nanoparticles in large alternating magnetic fields. Sci. Rep. 2011, 1, 157. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.; Khurshid, H.; Sankar, V.; Nemati, Z.; Phan, M.H.; Garayo, E.; García, J.A.; Srikanth, H. FeCo nanowires with enhanced heating powers and controllable dimensions for magnetic hyperthermia. J. Appl. Phys. 2015, 117, 17D113. [Google Scholar] [CrossRef]
- Usov, N.A.; Nesmeyanov, M.S.; Tarasov, V.P. Magnetic Vortices as Efficient Nano Heaters in Magnetic Nanoparticle Hyperthermia. Sci. Rep. 2018, 8, 1224. [Google Scholar] [CrossRef]
- Ng, E.Y.K.; Kumar, S.D. Physical mechanism and modeling of heat generation and transfer in magnetic fluid hyperthermia through Néelian and Brownian relaxation: A review. BioMed. Eng. OnLine 2017, 16, 36. [Google Scholar] [CrossRef] [Green Version]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Etemadi, H.; Plieger, P.G. Magnetic Fluid Hyperthermia Based on Magnetic Nanoparticles: Physical Characteristics, Historical Perspective, Clinical Trials, Technological Challenges, and Recent Advances. Adv. Ther. 2020, 3, 2000061. [Google Scholar] [CrossRef]
- Tong, S.; Zhu, H.; Bao, G. Magnetic iron oxide nanoparticles for disease detection and therapy. Mater. Today 2019, 31, 86–99. [Google Scholar] [CrossRef]
- Conde-Leboran, I.; Baldomir, D.; Martinez-Boubeta, C.; Chubykalo-Fesenko, O.; del Puerto Morales, M.; Salas, G.; Cabrera, D.; Camarero, J.; Teran, F.J.; Serantes, D. A Single Picture Explains Diversity of Hyperthermia Response of Magnetic Nanoparticles. J. Phys. Chem. C 2015, 119, 15698–15706. [Google Scholar] [CrossRef]
- Mehdaoui, B.; Meffre, A.; Carrey, J.; Lachaize, S.; Lacroix, L.M.; Gougeon, M.; Chaudret, B.; Respaud, M. Optimal Size of Nanoparticles for Magnetic Hyperthermia: A Combined Theoretical and Experimental Study. Adv. Funct. Mater. 2011, 21, 4573–4581. [Google Scholar] [CrossRef] [Green Version]
- Abenojar, E.C.; Wickramasinghe, S.; Bas-Concepcion, J.; Samia, A.C.S. Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles. Special Issue for Nano Materials. Prog. Nat. Sci. Mater. Int. 2016, 26, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Kafrouni, L.; Savadogo, O. Recent progress on magnetic nanoparticles for magnetic hyperthermia. Prog. Biomater. 2016, 5, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, J.; Xing, M.; Liu, J.P. Inductive Thermal Effect of Ferrite Magnetic Nanoparticles. Materials 2019, 12, 3208. [Google Scholar] [CrossRef] [Green Version]
- Aurélio, D.; Vejpravova, J. Understanding Magnetization Dynamics of a Magnetic Nanoparticle with a Disordered Shell Using Micromagnetic Simulations. Nanomaterials 2020, 10, 1149. [Google Scholar] [CrossRef]
- Pacakova, B.; Kubickova, S.; Salas, G.; Mantlikova, A.R.; Marciello, M.; Morales, M.P.; Niznansky, D.; Vejpravova, J. The internal structure of magnetic nanoparticles determines the magnetic response. Nanoscale 2017, 9, 5129–5140. [Google Scholar] [CrossRef]
- Serantes, D.; Simeonidis, K.; Angelakeris, M.; Chubykalo-Fesenko, O.; Marciello, M.; Morales, M.D.P.; Baldomir, D.; Martinez-Boubeta, C. Multiplying Magnetic Hyperthermia Response by Nanoparticle Assembling. J. Phys. Chem. C 2014, 118, 5927–5934. [Google Scholar] [CrossRef]
- Ranoo, S.; Lahiri, B.B.; Muthukumaran, T.; Philip, J. Enhancement in hyperthermia efficiency under in situ orientation of superparamagnetic iron oxide nanoparticles in dispersions. Appl. Phys. Lett. 2019, 115, 043102. [Google Scholar] [CrossRef]
- De la Presa, P.; Luengo, Y.; Multigner, M.; Costo, R.; Morales, M.P.; Rivero, G.; Hernando, A. Study of Heating Efficiency as a Function of Concentration, Size, and Applied Field in γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 2012, 116, 25602–25610. [Google Scholar] [CrossRef]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Fan, D.D.; Ding, J. Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Cobianchi, M.; Lascialfari, A.; Kusigerki, V.; Mrakovic, A.; Knezevic, N.; Peddis, D.; Illes, E. Effects of organic coating on hyperthermic efficiencies. In Proceedings of the 2017 IEEE International Magnetics Conference (INTERMAG), Dublin, Ireland, 24–28 April 2017. [Google Scholar] [CrossRef]
- Mikšátko, J.; Aurélio, D.; Kovaříček, P.; Michlová, M.; Veverka, M.; Fridrichová, M.; Matulková, I.; Žáček, M.; Kalbáč, M.; Vejpravová, J. Thermoreversible magnetic nanochains. Nanoscale 2019, 11, 16773–16780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torati, S.R.; Reddy, V.; Yoon, S.S.; Kim, C. Protein immobilization onto electrochemically synthesized CoFe nanowires. Int. J. Nanomed. 2015, 10, 645–651. [Google Scholar]
- Ruiz, A.; Alpízar, A.; Beola, L.; Rubio, C.; Gavilán, H.; Marciello, M.; Rodríguez-Ramiro, I.; Ciordia, S.; Morris, C.J.; Morales, M.D.P.; et al. Understanding the Influence of a Bifunctional Polyethylene Glycol Derivative in Protein Corona Formation around Iron Oxide Nanoparticles. Materials 2019, 12, 2218. [Google Scholar] [CrossRef] [Green Version]
- Vandenberghe, R.; Vanleerberghe, R.; De Grave, E.; Robbrecht, G. Preparation and magnetic properties of ultra-fine cobalt ferrites. J. Magn. Magn. Mater. 1980, 15–18, 1117–1118. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, D.; Lee, C.S. Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled coprecipitation method. Phys. B Condens. Matter 2003, 337, 42–51. [Google Scholar] [CrossRef]
- Carp, O.; Patron, L.; Reller, A. Coordination compounds containing urea as precursors for oxides—A new route of obtaining nanosized CoFe2O4. Mater. Chem. Phys. 2007, 101, 142–147. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Haneda, K.; Morrish, A.H. Noncollinear magnetic structure of CoFe2O4 small particles. J. Appl. Phys. 1988, 63, 4258–4260. [Google Scholar] [CrossRef]
- Salunkhe, A.; Khot, V.; Thorat, N.; Phadatare, M.; Sathish, C.; Dhawale, D.; Pawar, S. Polyvinyl alcohol functionalized cobalt ferrite nanoparticles for biomedical applications. Appl. Surf. Sci. 2013, 264, 598–604. [Google Scholar] [CrossRef]
- Baldi, G.; Bonacchi, D.; Franchini, M.C.; Gentili, D.; Lorenzi, G.; Ricci, A.; Ravagli, C. Synthesis and Coating of Cobalt Ferrite Nanoparticles: A First Step toward the Obtainment of New Magnetic Nanocarriers. Langmuir 2007, 23, 4026–4028. [Google Scholar] [CrossRef]
- Repko, A.; Nižnanský, D.; Poltierová-Vejpravová, J. A study of oleic acid-based hydrothermal preparation of CoFe2O4 nanoparticles. J. Nanoparticle Res. 2011, 13, 5021. [Google Scholar] [CrossRef]
- Sanna Angotzi, M.; Musinu, A.; Mameli, V.; Ardu, A.; Cara, C.; Niznansky, D.; Xin, H.L.; Cannas, C. Spinel Ferrite CoreShell Nanostructures by a Versatile Solvothermal Seed-Mediated Growth Approach and Study of Their Nanointerfaces. ACS Nano 2017, 11, 7889–7900. [Google Scholar] [CrossRef]
- van Rijssel, J.; Kuipers, B.W.; Erné, B.H. Non-regularized inversion method from light scattering applied to ferrofluid magnetization curves for magnetic size distribution analysis. J. Magn. Magn. Mater. 2014, 353, 110–115. [Google Scholar] [CrossRef]
- nanoScale Biomagnetics Official Webpage. Available online: https://www.nbnanoscale.com/ (accessed on 19 February 2021).
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. Physics responsible for heating efficiency and self-controlled temperature rise of magnetic nanoparticles in magnetic hyperthermia therapy. Prog. Biophys. Mol. Biol. 2018, 133, 9–19. [Google Scholar] [CrossRef]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Bruvera, I.J.; Mendoza Zélis, P.; Pilar Calatayud, M.; Goya, G.F.; Sánchez, F.H. Determination of the blocking temperature of magnetic nanoparticles: The good, the bad, and the ugly. J. Appl. Phys. 2015, 118, 184304. [Google Scholar] [CrossRef]
- Pacakova, B.; Mantlikova, A.; Niznansky, D.; Kubickova, S.; Vejpravova, J. Understanding particle size and distance driven competition of interparticle interactions and effective single-particle anisotropy. J. Physics: Condens. Matter 2016, 28, 206004. [Google Scholar] [CrossRef] [PubMed]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperth. 2019, 36, 686–700. [Google Scholar] [CrossRef] [PubMed]




| TEM (nm) | DLS (nm) | XRD (nm) | TGA (% of Coating) | |
|---|---|---|---|---|
| CoFe1_OA | ||||
| CoFe_MA | ||||
| CoFe_FU | ||||
| CoFe2_OA |
| 10K (T) | 10K () | 300 K () | / 10 K | (K) | |
|---|---|---|---|---|---|
| CoFe1_OA | 87 | 70 | 142 | ||
| CoFe_MA | 65 | 54 | 169 | ||
| CoFe_FU | 74 | 61 | 177 | ||
| CoFe2_OA | 91 | 75 | 214 |
() | (nm) | (nm) | () | () | |
|---|---|---|---|---|---|
| CoFe1_OA | |||||
| CoFe_MA | |||||
| CoFe_FU | |||||
| CoFe2_OA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aurélio, D.; Mikšátko, J.; Veverka, M.; Michlová, M.; Kalbáč, M.; Vejpravová, J. Thermal Traits of MNPs under High-Frequency Magnetic Fields: Disentangling the Effect of Size and Coating. Nanomaterials 2021, 11, 797. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11030797
Aurélio D, Mikšátko J, Veverka M, Michlová M, Kalbáč M, Vejpravová J. Thermal Traits of MNPs under High-Frequency Magnetic Fields: Disentangling the Effect of Size and Coating. Nanomaterials. 2021; 11(3):797. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11030797
Chicago/Turabian StyleAurélio, David, Jiří Mikšátko, Miroslav Veverka, Magdalena Michlová, Martin Kalbáč, and Jana Vejpravová. 2021. "Thermal Traits of MNPs under High-Frequency Magnetic Fields: Disentangling the Effect of Size and Coating" Nanomaterials 11, no. 3: 797. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11030797