Elasticity of the Cervix in Relation to Uterus Position
Abstract
:1. Introduction
2. Materials and Methods
3. Results
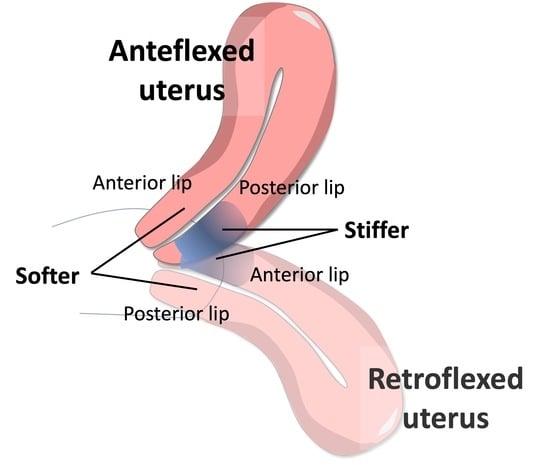
3.1. Elasticity of the Cervix
3.2. Multiple Regression Models
3.3. Data Stratification by Angle of Uterine Flexion
4. Discussion
4.1. Principal Findings
4.2. Results in the Context of What Is Known
4.3. Clinical Implications
4.4. Research Implications
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minamoto, T.; Arai, K.; Hirakawa, S.; Nagai, Y. Immunohistochemical studies on collagen types in the uterine cervix in pregnant and nonpregnant states. Am. J. Obstet. Gynecol. 1987, 156, 138–144. [Google Scholar] [CrossRef]
- Aspden, R.M. Collagen organisation in the cervix and its relation to mechanical function. Coll. Relat. Res. 1988, 8, 103–112. [Google Scholar] [CrossRef]
- Vink, J.Y.; Qin, S.; Brock, C.O.; Zork, N.M.; Feltovich, H.M.; Chen, X.; Urie, P.; Myers, K.M.; Hall, T.J.; Wapner, R.; et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am. J. Obstet. Gynecol. 2016, 215, 478.e1–478.e11. [Google Scholar] [CrossRef]
- Gan, Y.; Yao, W.; Myers, K.M.; Vink, J.Y.; Wapner, R.J.; Hendon, C.P. Analyzing three-dimensional ultrastructure of human cervical tissue using optical coherence tomography. Biomed. Opt. Express 2015, 6, 1090–1108. [Google Scholar] [CrossRef]
- Yao, W.; Gan, Y.; Myers, K.M.; Vink, J.Y.; Wapner, R.J.; Hendon, C.P. Collagen Fiber Orientation and Dispersion in the Upper Cervix of Non-Pregnant and Pregnant Women. PLoS ONE 2016, 11, e0166709. [Google Scholar] [CrossRef] [PubMed]
- Ateshian, G.A. Anisotropy of fibrous tissues in relation to the distribution of tensed and buckled fibers. J. Biomech. Eng. 2007, 129, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Ateshian, G.A.; Rajan, V.; Chahine, N.O.; Canal, C.E.; Hung, C.T. Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena. J. Biomech. Eng. 2009, 131, 061003. [Google Scholar] [CrossRef]
- Fernandez, M.; House, M.; Jambawalikar, S.; Zork, N.; Vink, J.; Wapner, R.; Myers, K. Investigating the mechanical function of the cervix during pregnancy using finite element models derived from high-resolution 3D MRI. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Louwagie, E.M.; Carlson, L.; Over, V.; Mao, L.; Fang, S.; Westervelt, A.; Vink, J.; Hall, T.; Feltovich, H.; Myers, K. Longitudinal ultrasonic dimensions and parametric solid models of the gravid uterus and cervix. PLoS ONE 2021, 16, e0242118. [Google Scholar] [CrossRef]
- Myers, K.M.; Paskaleva, A.P.; House, M.; Socrate, S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008, 4, 104–116. [Google Scholar] [CrossRef]
- House, M.; Socrate, S. The cervix as a biomechanical structure. Ultrasound Obstet. Gynecol. 2006, 28, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.M.; Feltovich, H.; Mazza, E.; Vink, J.; Bajka, M.; Wapner, R.J.; Hall, T.J.; House, M. The mechanical role of the cervix in pregnancy. J. Biomech. 2015, 48, 1511–1523. [Google Scholar] [CrossRef]
- Huebner, M.; DeLancey, J.O.L. Levels of pelvic floor support: What do they look like on magnetic resonance imaging? Int. Urogynecol. J. 2019, 30, 1593–1595. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yao, W.; Gan, Y.; Zhao, L.Y.; McKee, W.E.; Vink, J.; Wapner, R.J.; Hendon, C.P.; Myers, K. Anisotropic Material Characterization of Human Cervix Tissue Based on Indentation and Inverse Finite Element Analysis. J. Biomech. Eng. 2019, 141, 0910171–0910173. [Google Scholar] [CrossRef] [PubMed]
- Molina, F.S.; Gómez, L.F.; Florido, J.; Padilla, M.C.; Nicolaides, K.H. Quantification of cervical elastography: A reproducibility study. Ultrasound Obstet. Gynecol. 2012, 39, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Hee, L.; Rasmussen, C.K.; Schlütter, J.M.; Sandager, P.; Uldbjerg, N. Quantitative sonoelastography of the uterine cervix prior to induction of labor as a predictor of cervical dilation time. Acta Obstet. Gynecol. Scand. 2014, 93, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Feltovich, H. Cervical Evaluation: From Ancient Medicine to Precision Medicine. Obstet. Gynecol. 2017, 130, 51–63. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Hernandez-Andrade, E.; Hassan, S.S.; Ahn, H.; Kozzeniewski, S.J.; Yeo, L.; Chaiworapongsa, T.; Romero, R. Evaluation of cervical stiffness during pregnancy using semiquantitative ultrasound elastography. Ultrasound Obstet. Gynecol. 2013, 41, 152–161. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Londero, A.P.; Fröhlich, C.; Möllmann, U.; Schmitz, R. Quantitative elastography for cervical stiffness assessment during pregnancy. Biomed. Res. Int. 2014, 2014, 826535. [Google Scholar] [CrossRef]
- Hernandez-Andrade, E.; Garcia, M.; Ahn, H.; Korzeniewsky, S.J.; Saker, H.; Yeo, L.; Chaiworapongsa, T.; Hassan, S.S.; Romero, R. Strain at the internal cervical os assessed with quasi-static elastography is associated with the risk of spontaneous preterm delivery at ≤34 weeks of gestation. J. Perinat. Med. 2015, 43, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Chen, S.; Xiang, X.; Wen, J.; Yi, M.; He, B.; Hu, B. Diagnostic accuracy of cervical elastography in predicting preterm delivery: A systematic review and meta-analysis. Medicine 2019, 98, e16449. [Google Scholar] [CrossRef] [PubMed]
- Stanziano, A.; Caringella, A.M.; Cantatore, C.; Trojano, G.; Caroppo, E.; D’Amato, G. Evaluation of the cervix tissue homogeneity by ultrasound elastography in infertile women for the prediction of embryo transfer ease: A diagnostic accuracy study. Reprod. Biol. Endocrinol. 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Xholli, A.; Simoncini, G.; Vujosevic, S.; Trombetta, G.; Chiodini, A.; Ferraro, M.F.; Cagnacci, A. Menstrual Pain and Elasticity of Uterine Cervix. J. Clin. Med. 2021, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Grandi, G.; Cannoletta, M.; Xholli, A.; Piacenti, I.; Volpe, A. Intensity of menstrual pain and estimated angle of uterine flexion. Acta Obstet. Gynecol. Scand. 2014, 93, 58–63. [Google Scholar] [CrossRef]
- Xholli, A.; Scovazzi, U.; Londero, A.P.; Evangelisti, G.; Cavalli, E.; Schiaffino, M.G.; Vacca, I.; Oppedisano, F.; Ferraro, M.F.; Sirito, G.; et al. Angle of Uterine Flexion and Adenomyosis. J. Clin. Med. 2022, 11, 3214. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, T.; Leo, L.; Gomel, V. Laparoscopic uterine suspension using three-stitch technique. J. Am. Assoc. Gynecol. Laparosc. 2000, 7, 233–236. [Google Scholar] [CrossRef]
- Ott, J.; Nouri, K.; Demmel, M.; Zafraani, S.; Greilberger, U.; Huber, J.C.; Mayerhofer, K. Fourteen-year experience with laparoscopic ventrosuspension in patients with retroverted and retroflected uterus and pelvic pain syndromes. J. Minim. Invasive Gynecol. 2010, 17, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Seracchioli, R.; Zanello, M.; Arena, A.; Costantino, C.; Moro, E.; Zannoni, L.; Raimondo, D. New Laparoscopic Technique of Hysteropexy for Uterine Retrodisplacement: Bologna Technique. J. Minim. Invasive Gynecol. 2016, 23, 675. [Google Scholar] [CrossRef]
- Xholli, A.; Molinari, F.; Oppedisano, F.; Scovazzi, U.; Vacca, I.; Schiaffino, M.G.; Cavalli, E.; Cagnacci, A. Relation between adenomyosis and elastographic characteristics of the cervix. Hum. Reprod. 2023, 38, 621–628. [Google Scholar] [CrossRef]
- Dziadosz, M.; Bennett, T.A.; Dolin, C.; West Honart, A.; Pham, A.; Lee, S.S.; Pivo, S.; Roman, A.S. Uterocervical angle: A novel ultrasound screening tool to predict spontaneous preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 376.e1–376.e7. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Value |
|---|---|
| Age (yrs.) | 36.7 ± 7.5 |
| Menarche (yrs.) | 12.6 ± 1.6 |
| BMI (Kg/m2) | 22.9 ± 4.8 |
| Abortion (%) | 4.1 |
| Parous (%) | 20.5 |
| Caesarean delivery (%) | 4.5 |
| Uterus Volume (mm3) | 61.9 ± 45.3 |
| Uterus L (mm) | 59.5 ± 13.6 |
| Uterus AP (mm) | 39.0 ± 10.4 |
| Uterus T (mm) | 50.9 ± 10.5 |
| Cervix volume (mm3) | 20.1 ± 6.18 |
| Cervix length (mm) | 26.6 ± 5.19 |
| Cervix diameter (mm) | 23.5 ± 4.9 |
| ACC elasticity | 0.94 ± 0.36 |
| PCC elasticity | 0.74 ± 0.29 |
| MCC elasticity | 1.39 ± 0.51 |
| ICO elasticity | 0.63 ± 0.28 |
| Parameter | R2 | Coefficient of Regression | 95% Confidence Interval | p Value |
|---|---|---|---|---|
| Age (yrs.) | 0.013 | 0.828 | 0.054; 1.601 | 0.036 |
| Uterus Volume (mm3) | 0.021 | 0.173 | 0.042; 0.303 | 0.009 |
| Uterus AP (mm) | 0.040 | 0.970 | 0.421; 1.520 | 0.001 |
| Uterus T (mm) | 0.017 | 0.670 | 0.116; 1.225 | 0.018 |
| Cervix Diameter (mm) | 0.010 | −1.175 | −2.376; 0.002 | 0.059 |
| Uterus AP/Cervix diameter | 0.065 | 23.67 | 13.09; 34.28 | 0.001 |
| ACC elasticity | 0.071 | −36.2 | −51.51; −20.913 | 0.002 |
| PCC elasticity | 0.010 | 19.6 | −0.360; 39.710 | 0.054 |
| MCC elasticity | 0.047 | −26.611 | −32.81; −10.40 | 0.002 |
| ACC/PCC | 0.490 | −11.34 | −17.68; −5.60 | 0.001 |
| PCC/MCC | 0.109 | 28.44 | 18.87; 38.02 | 0.001 |
| Parameter | Coefficient of Regression | 95% Confidence Interval | p Value |
|---|---|---|---|
| Model 1 (R2 = 0.116) | |||
| Uterus AP (mm) | 1.014 | 0.461; 1.567 | 0.001 |
| Cervix Diameter (mm) | −1.24 | −2.401; −0.075 | 0.037 |
| ACC color score | −33.9 | −49.2; −18.65 | 0.001 |
| Model 2 (R2 = 0.124) | |||
| Uterus AP/Cervix diameter | 21.66 | 11.34; 31.89 | 0.001 |
| ACC color score | −33.4 | −48.6; −18.2 | 0.001 |
| Model 3 (R2 = 0.163) | |||
| Uterus AP/Cervix diameter | 21.67 | 11.60; 31.75 | 0.001 |
| PCC/MCC | 26.83 | 17.41; 32.21 | 0.001 |
| Parameter | A | B | C | p A vs. B | p A vs. C | p B vs. C |
|---|---|---|---|---|---|---|
| Age (yrs.) | 35.3 ± 7.1 | 41.8 ± 7.1 | 34.7 ± 8.2 | 0.001 | 0.632 | 0.004 |
| Menarche (Yrs.) | 12.5 ± 1.7 | 12.8 ± 1.5 | 12.7 ± 1.2 | 0.723 | 0.718 | 0.907 |
| BMI (Kg/m2) | 22.7 ± 4.8 | 24.6 ± 5.2 | 23.1 ± 4.7 | 0.131 | 0.692 | 0.288 |
| Uterus Volume (mm3) | 56.7 ± 35.40 | 86.1 ± 86.9 | 65.9 ± 51.1 | 0.004 | 0.232 | 0.089 |
| Uterus L (mm) | 54.0 ± 11.4 | 56.8 ± 21.6 | 53.7 ± 14.80 | 0.356 | 0.890 | 0.383 |
| Uterus AP (mm) | 37.7 ± 8.8 | 48.4 ± 16.1 | 40.4 ± 11.8 | 0.001 | 0.132 | 0.004 |
| Uterus T (mm) | 50.2 ± 9.7 | 56.8 ± 16.6 | 51.3 ± 10.1 | 0.007 | 0.520 | 0.060 |
| Cervix volume (mm3) | 19.8 ± 5.8 | 22.0 ± 8.5 | 19.9 ± 6.0 | 0.115 | 0.090 | 0.016 |
| Cervix Diameter (mm) | 23.9 ± 4.6 | 23.4 ± 6.5 | 21.6 ± 5.2 | 0.693 | 0.009 | 0.182 |
| ACC elasticity | 0.98 ± 0.37 | 0.89 ± 0.3 | 0.69 ± 0.26 | 0.239 | 0.001 | 0.044 |
| PCC elasticity | 0.72 ± 0.290 | 0.71 ± 0.25 | 0.86 ± 0.24 | 0.859 | 0.004 | 0.046 |
| MCC elasticity | 1.44 ± 0.51 | 1.44 ± 0.59 | 1.08 ± 0.36 | 0.976 | 0.001 | 0.010 |
| ICO elasticity | 0.62 ± 0.28 | 0.58 ± 0.29 | 0.66 ± 0.23 | 0.442 | 0.425 | 0.246 |
| ACC/PCC | 1.62 ± 1.03 | 1.39 ± 0.77 | 0.90 ± 0.53 | 0.292 | 0.001 | 0.058 |
| PCC/MCC | 0.83 ± 0.49 | 0.89 ± 0.41 | 1.45 ± 0.76 | 0.612 | 0.001 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xholli, A.; Londero, A.P.; Scovazzi, U.; Cagnacci, A. Elasticity of the Cervix in Relation to Uterus Position. J. Clin. Med. 2024, 13, 2572. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13092572
Xholli A, Londero AP, Scovazzi U, Cagnacci A. Elasticity of the Cervix in Relation to Uterus Position. Journal of Clinical Medicine. 2024; 13(9):2572. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13092572
Chicago/Turabian StyleXholli, Anjeza, Ambrogio Pietro Londero, Umberto Scovazzi, and Angelo Cagnacci. 2024. "Elasticity of the Cervix in Relation to Uterus Position" Journal of Clinical Medicine 13, no. 9: 2572. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13092572