Long-Term Postnatal Follow-Up in Monochorionic TTTS Twin Pregnancies Treated with Fetoscopic Laser Surgery and Complicated by Right Ventricular Outflow Tract Anomalies
Abstract
:1. Introduction
2. Materials and Methods
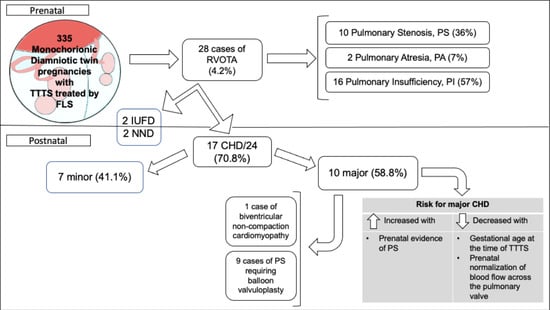
3. Results
| Case N | RVOTA | Twin with RVOTA | Onset RVOTA | Severe TV-R | Severe MV-R | DV a-Wave | C/T Ratio ≥ 0.55 | TTTS | Additional US Findings Detected during Pregnancy | Last US (Weeks) | Normalization FVW-PV | Pregnancy Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | GA (Weeks) | Type | GA (Weeks) | |||||||||||
| 1 | PS | 15.0 | R | Before TTTS | yes | yes | no | yes | 3 | 15.6 | IUD D 24 h after FLS. Biventricular hypertrophy | 21.6 | no | Alive |
| 2 | PS | 20.3 | R | After FLS | yes | no | yes | yes | 3 | 18.2 | None | 35.2 | no | Alive |
| 3 | PS | 19.6 | R | Before TTTS | no | no | yes | no | 1 | 21.4 | None | 26.0 | no | Alive |
| 4 | PI | 20.6 | R | At the time of TTTS | yes | no | no | yes | 4 | 20.6 | PA at 23 weeks | 24.1 | no | IUD |
| 5 | PA | 21.0 | R | At the time of TTTS | yes | no | no | yes | 4 | 21.0 | Dilated cardiomyopathy | NA | yes | Alive |
| 6 | PI | 21.1 | D | After FLS | yes | no | no | no | 2 | 20.0 | Ex-donor tricuspid valve dysplasia 11 weeks after FLS | 31.1 | yes | Alive |
| 7 | PS | 23.3 | R | After FLS | no | no | yes | no | 2 | 17.6 | PS 5 weeks after FLS | 23.3 | no | Alive |
| 8 | PI | 21.3 | R | At the time of TTTS | yes | yes | no | yes | 4 | 21.3 | None | 27.0 | yes | Alive |
| 9 | PS | 17.3 | R | At the time of TTTS | yes | yes | no | yes | 3 | 17.3 | IUD donor 24 h after FLS. | 30.3 | no | Alive |
| 10 | PI | 23.5 | D | After FLS | yes | no | no | yes | 2 | 22.5 | Ex-donor hydrops due to heart failure 6 days after FLS | 33.1 | yes | Alive |
| 11 | PS | 23.0 | R | At the time of TTTS | yes | yes | no | yes | 4 | 23.0 | Myocardial hypertrophy | 35.3 | no | Alive |
| 12 | PA | 23.6 | D | After FLS | no | no | yes | no | 2 | 21.5 | Ex-donor: hydrops due to heart failure 13 days after FLS; therapy with Digoxin from 26 weeks. Mirror syndrome | 30.0 | yes | Alive |
| 13 | PS | 24.0 | R | After FLS | yes | no | yes | yes | 1 | 20.1 | TTTS recurrence 3 weeks after FLS; amniodecompression | 33.0 | yes | Alive |
| 14 | PS | 18.2 | R | After FLS | yes | no | yes | yes | 2 | 16.3 | Ex-donor IUD 14 days after FLS | 31.0 | yes | Alive |
| 15 | PI | 22.4 | D | After FLS | no | no | no | yes | 2 | 19.6 | Hydrops due to heart failure 12 days after FLS, spontaneously resolved | 34.4 | yes | Alive |
| 16 | PS | 17.4 | R | At the time of TTTS | yes | no | yes | yes | 2 | 17.4 | Ex-donor sFGR with AEDF | 30.1 | no | Alive |
| 17 | PS | 26.2 | R | After FLS | no | yes | yes | yes | 3 | 21.0 | IUD ex-donor sFGR with REDF. Myocardial hypertrophy | 27.3 | yes | Alive |
| 18 | PI | 26.3 | R | Before TTTS | no | no | no | yes | 3 | 26.4 | IUD ex-donor 14 days after FLS | 34.1 | yes | Alive |
| 19 | PI | 20.0 | R | After FLS | yes | yes | no | yes | 2 | 19.0 | TTTS persistence after FLS; 2° FLS after 7 days. Heart failure in ex-recipient with hydrops; ex-donor with sFGR | 22.1 | no | TOP (recurrence of TTTS with hydrops of the recipient and donor severe sFGR with REDF in UA) |
| 20 | PI | 24.4 | R | At the time of TTTS | yes | no | no | yes | 4 | 24.4 | None | 26.0 | yes | Alive |
| 21 | PI | 19.5 | R | After FLS | yes | yes | no | yes | 1 | 18.5 | Ascites 24 h after FLS | 34.5 | yes | Alive |
| 22 | PI | 17.4 | R | At the time of TTTS | yes | no | no | yes | 4 | 17.4 | Myocardial hypertrophy | 31.1 | yes | Alive |
| 23 | PI | 25.1 | R | At the time of TTTS | yes | no | no | yes | 4 | 25.1 | MRI before FLS: IVH grade 1 | 30.0 | yes | Alive |
| 24 | PI | 22.2 | R | At the time of TTTS | yes | no | no | yes | 4 | 22.2 | Myocardial hypertrophy | 25.3 | yes | Alive |
| 25 | PI | 22.6 | R | After FLS | yes | no | no | yes | 2 | 22.0 | Ex-donor with SNC damage. Selective TOP of ex-donor. Amniodecompression 2 days after FLS. Myocardial hypertrophy. | 23.2 | yes | Alive |
| 26 | PI | 20.4 | R | At the time of TTTS | yes | yes | no | yes | 3 | 20.4 | TAPS sequence 48 h after FSL. Myocardial hypertrophy | 22.6 | no | Alive |
| 27 | PI | 26.3 | R | After FLS | no | no | no | no | 1 | 20.1 | TTTS recurrence 6 weeks after FLS; 3 amniodecompression; heart failure of ex-recipient treated with Digoxin from 26 weeks. Biventricular hypokinesia | 28.5 | no | Alive |
| 28 | PI | 23.4 | R | At the time of TTTS | yes | yes | no | yes | 4 | 23.4 | Ex-recipient heart failure from 26 weeks treated with Digoxin; ex-donor sFGR. Myocardial hypertrophy | 33.0 | yes | Alive |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lougheed, J.; Sinclair, B.G.; Fung, K.F.K.; Bigras, J.-L.; Ryan, G.; Smallhorn, J.F.; Hornberger, L.K. Acquired right ventricular outflow tract obstruction in the recipient twin in twin-twin transfusion syndrome. J. Am. Coll. Cardiol. 2001, 38, 1533–1538. [Google Scholar] [CrossRef]
- Karatza, A.A.; Wolfenden, J.L.; Taylor, M.J.; Wee, L.; Fisk, N.M.; Gardiner, H.M. Influence of twin-twin transfusion syndrome on fetal cardiovascular structure and function: Prospective case-control study of 136 monochorionic twin pregnancies. Heart 2002, 88, 271–277. [Google Scholar] [CrossRef]
- Fisk, N.M.; Duncombe, G.J.; Sullivan, M.H.F. The basic and clinical science of twin–twin transfusion syndrome. Placenta 2009, 30, 379–390. [Google Scholar] [CrossRef]
- Bajoria, R.; Ward, S.; Sooranna, S.R. Atrial natriuretic peptide mediated polyuria: Pathogenesis of polyhydramnios in the recipient twin of twin-twin transfusion syndrome. Placenta 2001, 22, 716–724. [Google Scholar] [CrossRef]
- Senat, M.V.; Deprest, J.; Boulvain, M.; Paupe, A.; Winer, N.; Ville, Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N. Engl. J. Med. 2004, 351, 136–144. [Google Scholar] [CrossRef]
- Moon-Grady, A.J.; Rand, L.; Lemley, B.; Gosnell, K.; Hornberger, L.K.; Lee, H. Effect of selective fetoscopic laser photocoagulation therapy for twin-twin transfusion syndrome on pulmonary valve pathology in recipient twins. Ultrasound Obstet. Gynecol. 2011, 37, 27–33. [Google Scholar] [CrossRef]
- Eschbach, S.J.; Ten Harkel, A.D.J.; Middeldorp, J.M.; Klumper, F.J.C.M.; Oepkes, D.; Lopriore, E.; Haak, M.C. Acquired Right Ventricular Outflow Tract Obstruction in twin-to-twin transfusion syndrome; a prospective longitudinal study. Prenat. Diagn. 2018, 38, 1013–1019. [Google Scholar] [CrossRef]
- Quintero, R.A.; Morales, W.J.; Alien, M.H.; Bomik, P.W.; Johnson, P.K.; Kruger, M. Staging of twin-twin transfusion syndrome. J. Perinatol. 1999, 19, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Michelfelder, E.; Tan, X.; Cnota, J.; Divanovic, A.; Statile, C.; Lim, F.-Y.; Crombleholme, T. Prevalence, Spectrum, and Outcome of Right Ventricular Outflow Tract Abnormalities in Twin-twin Transfusion Syndrome: A Large, Single-center Experience. Congenit. Heart Dis. 2015, 10, 209–218. [Google Scholar] [CrossRef]
- Slaghekke, F.; Lopriore, E.; Lewi, L.; Middeldorp, J.M.; van Zwet, E.W.; Weingertner, A.S.; Klumper, F.J.; DeKoninck, P.; Devlieger, R.; Kilby, M.D.; et al. Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin-to-twin transfusion syndrome: An open-label randomised controlled trial. Lancet 2014, 383, 2144–2151. [Google Scholar] [CrossRef]
- Cantinotti, M.; Giordano, R.; Emdin, M.; Assanta, N.; Crocetti, M.; Marotta, M.; Iervasi, G.; Lopez, L.; Kutty, S. Echocardiographic assessment of pediatric semilunar valve disease. Echocardiography 2017, 34, 1360–1370. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.; Pellikka, P.A.; Quiñones, M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 2009, 10, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Van Mieghem, T.; Klaritsch, P.; Doné, E.; Gucciardo, L.; Lewi, P.; Verhaeghe, J.; Lewi, L.; Deprest, J. Assessment of fetal cardiac function before and after therapy for twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2009, 200, 400.e1–400.e7. [Google Scholar] [CrossRef]
- Chang, Y.L.; Chao, A.S.; Chang, S.D.; Cheng, P.J.; Li, W.F.; Hsu, C.C. Incidence, prognosis, and perinatal outcomes of and risk factors for severe twin-twin transfusion syndrome with right ventricular outflow tract obstruction in the recipient twin after fetoscopic laser photocoagulation. BMC Pregnancy Childbirth 2022, 22, 326. [Google Scholar] [CrossRef]
- Faiola, S.; Casati, D.; Laoreti, A.; Amendolara, M.; Consonni, D.; Corti, C.; Mannarino, S.; Lanna, M.; Rustico, M.; Cetin, I. Right ventricular outflow tract abnormalities in monochorionic twin pregnancies without twin-to-twin transfusion syndrome: Prenatal course and postnatal long-term outcomes. Prenat. Diagn. 2021, 41, 1510–1517. [Google Scholar] [CrossRef]
- Faiola, S.; Casati, D.; Nelva Stellio, L.; Laoreti, A.; Corti, C.; Mannarino, S.; Lanna, M.; Cetin, I. Congenital heart defects in monochorionic twin pregnancy complicated by selective fetal growth restriction. Ultrasound Obstet. Gynecol. 2023, 61, 504–510. [Google Scholar] [CrossRef]
- Eschbach, S.J.; Boons, L.S.T.M.; Van Zwet, E.; Middeldorp, J.M.; Klumper, F.J.C.M.; Lopriore, E.; Teunissen, A.K.K.; Rijlaarsdam, M.E.; Oepkes, D.; Ten Harkel, A.D.J.; et al. Right ventricular outflow tract obstruction in complicated monochorionic twin pregnancy. Ultrasound Obstet. Gynecol. 2017, 49, 737–743. [Google Scholar] [CrossRef]
- Faiola, S.; Casati, D.; Laoreti, A.; Corti, C.; Bianchi, S.; Nelva Stellio, L.; Cetin, I.; Lanna, M. Birth prevalence of right ventricular outflow tract abnormalities in the recipient twin of monochorionic twin pregnancies complicated by twin-to-twin transfusionsyndrome: Amnioreduction versus fetoscopic laser coagulation treatment. Ital. J. Gynæcology Obstet. 2023, 35, 190–195. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; de la Pompa, J.L. Left ventricular noncompaction: A genetic cardiomyopathy looking for diagnostic criteria. J. Am. Coll. Cardiol. 2014, 64, 1981–1983. [Google Scholar] [CrossRef] [Green Version]
- Jenni, R.; Rojas, J.; Oechslin, E. Isolated noncompaction of the myocardium. N. Engl. J. Med. 1999, 340, 966–967. [Google Scholar] [CrossRef]
- Rojanasopondist, P.; Nesheiwat, L.; Piombo, S.; Porter GAJr Ren, M.; Phoon, C.K.L. Genetic Basis of Left Ventricular Noncompaction. Circ. Genom. Precis. Med. 2022, 15, 190–200. [Google Scholar] [CrossRef] [PubMed]

| Case N * | GA at the Delivery | BW (gr) | Neonatal Type of RVOTA | Years of Follow-Up | CHD at Follow-Up |
|---|---|---|---|---|---|
| 1 | 32 | 1850 | PS | 4 | Major CHD: severe PS with BV |
| 2 | 35 | 2960 | PS | 8 | Major CHD: severe PS with BV and closure of the ductus arteriosus |
| 3 | 33 | 1500 | PA | 7 | Major CHD: PA with intact ventricular septum, BV, iatrogenic hemopericardium during the procedure. Persistence of mild PI; possibility of re-intervention |
| 5 | 36 | 1960 | 0 | 8 | Minor CHD: left ventricular hypertrabeculation |
| 6 | 34 | 1490 | 0 | 9 | Minor CHD: tricuspid dysplasia with mild to moderate TV-R |
| 7 | 34 | 2020 | PS | 5 | Major CHD: severe PS with BV. Residual PSI at FU |
| 8 | 34 | 1690 | 0 | 6 | None |
| 9 | 37 | 2680 | PS | 11 | Major CHD: severe PS with BV |
| 10 | 33 | 1793 | 0 | 10 | None |
| 11 | 35 | 2170 | PS | 11 | Major CHD: severe PS with BV, atrial septal defect closure. Planned replacement of the PV due to the residual severe PI |
| 12 | 30 | 1200 | 0 | 11 | Minor CHD: neonatal correction of patent ductus arteriosus; dysplastic atrioventricular valves; persistence of moderate TV-R and MV-R at the follow-up, without the need for surgical correction |
| 13 | 33 | 2150 | 0 | 10 | Minor CHD: tricuspid dysplasia with moderate TV-R |
| 14 | 38 | 2700 | PS | 10 | Minor CHD: mild PSI, gradient 34 mmHg. Stationary follow-up |
| 15 | 35 | 2340 | 0 | 12 | None |
| 16 | 31 | 1630 | PS | 10 | Major CHD: severe PS with BV; hypertrophic cardiomyopathy |
| 17 | 28 | 1430 | NA | 11 | None |
| 18 | 35 | 2285 | 0 | 3 | None |
| 20 | 26 | 1100 | NA | NA | NND due to severe prematurity at birth, severe RDS and heart failure with biventricular dilatation and systolic dysfunction |
| 21 | 34 | 2660 | 0 | 10 | None |
| 22 | 32 | 1700 | PS | 5 | Major CHD: severe PS with BV |
| 23 | 33 | 1800 | 0 | 6 | None |
| 24 | 26 | 1000 | PI | 4 | Minor CHD: mitral dysplasia with MV-R: no intervention |
| 25 | 24 | 500 | PS | 13 | Major CHD: severe PS with BV |
| 26 | 28 | 690 | 0 | 11 | Major CHD: cardiomyopathy at 7 years, non-compact myocardium |
| 27 | 28 | 990 | NA | NA | NND due to heart failure |
| 28 | 33 | 1900 | 0 | 9 | Minor CHD: tricuspid and mitral dysplasia with moderate TV-R and mild MV-R at birth |
| Variable | N or Median (Range) | Cases with Major CHDs N (%) or Median (Range) | Hazard Ratio (95%CI) |
|---|---|---|---|
| Pulmonary insufficiency | 12 | 3 (25) | 1.00 (Reference) |
| Pulmonary stenosis | 10 | 7 (70) | 4.71 (1.18–18.7) |
| Pulmonary atresia | 2 | 0 | NC |
| Recipient twin with an RVOTA | 20 | 10 (50) | 1.00 (Reference) |
| Donor twin with an RVOTA | 4 | 0 | NC |
| RVOTA at the time of TTTS | 10 | 5 (50) | 1.00 (Reference) |
| RVOTA before TTTS | 3 | 2 (67) | 1.48 (0.28–7.69) |
| RVOTA developed after FLS | 11 | 3 (27) | 0.52 (0.12–2.19) |
| Gestational age at time of TTTS (weeks) | 21.2 (16.4–23.6) | 18.1 (15.9–23) | 0.69 (0.50–0.97) |
| TTTS Stage 1–2 | 12 | 5 (42) | 1.00 (Reference) |
| TTTS Stage 3–4 | 12 | 5 (42) | 1.28 (0.37–4.43) |
| No selective fetal growth restriction | 12 | 5 (42) | 1.00 (Reference) |
| Selective fetal growth restriction | 12 | 5 (42) | 1.16 (0.33–4.04) |
| Cardio/thoracic ratio < 0.55 | 8 | 3 (37) | 1.00 (Reference) |
| Cardio/thoracic ratio ≥ 0.55 | 16 | 7 (43) | 1.49 (0.38–5.86) |
| No reversed a-wave in ductus venosus | 8 | 3 (37) | 1.00 (Reference) |
| Reversed a-wave in ductus venosus | 16 | 7 (43) | 1.25 (0.32–4.85) |
| No severe insufficiency in TV | 7 | 2 (29) | 1.00 (Reference) |
| Severe insufficiency in TV | 17 | 8 (47) | 1.62 (0.34–7.79) |
| No severe insufficiency in MV | 13 | 4 (31) | 1.00 (Reference) |
| Severe insufficiency in MV | 11 | 6 (54) | 2.23 (0.63–7.93) |
| No prenatal normalization of the flow across the pulmonary valve | 8 | 8 (100) | 1.00 (Reference) |
| Prenatal normalization of the flow across the pulmonary valve | 16 | 2 (12) | 0.09 (0.02–0.42) |
| Gestational age at delivery (weeks) | 33 (24–38) | 32 (24–35) | 0.89 (0.73–1.08) |
| Weight at delivery (gr) | 1.850 (500–2.960) | 1.770 (500–2170) | 0.99 (0.99–1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faiola, S.; Mandalari, M.; Coco, C.; Casati, D.; Laoreti, A.; Mannarino, S.; Corti, C.; Consonni, D.; Cetin, I.; Lanna, M. Long-Term Postnatal Follow-Up in Monochorionic TTTS Twin Pregnancies Treated with Fetoscopic Laser Surgery and Complicated by Right Ventricular Outflow Tract Anomalies. J. Clin. Med. 2023, 12, 4734. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12144734
Faiola S, Mandalari M, Coco C, Casati D, Laoreti A, Mannarino S, Corti C, Consonni D, Cetin I, Lanna M. Long-Term Postnatal Follow-Up in Monochorionic TTTS Twin Pregnancies Treated with Fetoscopic Laser Surgery and Complicated by Right Ventricular Outflow Tract Anomalies. Journal of Clinical Medicine. 2023; 12(14):4734. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12144734
Chicago/Turabian StyleFaiola, Stefano, Maria Mandalari, Chiara Coco, Daniela Casati, Arianna Laoreti, Savina Mannarino, Carla Corti, Dario Consonni, Irene Cetin, and Mariano Lanna. 2023. "Long-Term Postnatal Follow-Up in Monochorionic TTTS Twin Pregnancies Treated with Fetoscopic Laser Surgery and Complicated by Right Ventricular Outflow Tract Anomalies" Journal of Clinical Medicine 12, no. 14: 4734. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12144734