Luteolin Alleviates Cadmium-Induced Kidney Injury by Inhibiting Oxidative DNA Damage and Repairing Autophagic Flux Blockade in Chickens
Abstract
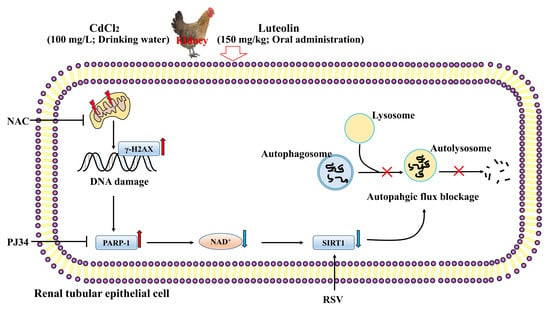
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Animals and Experimental Design
2.3. Extraction, Culture and Treatment of Chicken RTECs
2.4. Observation of Histopathology and Ultrastructure
2.5. Detection of Biochemical Parameters
2.6. Determination of Metal Concentrations
2.7. Measurement of ROS and Oxidative Stress Indexes
2.8. Detection of NAD+ and NADH Contents
2.9. Determination of 8-OHdG Content
2.10. Immunohistochemistry (IHC)
2.11. TdT-Mediated dUTP Nick-End Labeling (TUNEL) Staining
2.12. Western Blotting Analysis
2.13. Statistical Analysis
3. Results
3.1. Cd-Induced Oxidative DNA Damage, PARP-1 Over-Activation, Decreased SIRT1 Activity, and Autophagic Flux Blockade in Chicken RTECs
3.2. Alleviating Oxidative Stress Attenuated Cd-Induced PARP-1 Over-Activation, SIRT1 Inhibition, and Autophagic Flux Blocking in Chicken RTECs
3.3. Inhibition of PARP-1 Restored SIRT1 Activity and Autophagic Flux in Chicken RTECs
3.4. Lut Alleviates Cd-Induced Kidney Injury
3.5. Lut Alleviates Cd-Induced Oxidative Stress
3.6. Lut Attenuates Cd-Induced DNA Damage and PARP-1 Activation
3.7. Lut Restores Cd-Induced SIRT1 Inactivity and Autophagic Flux Blockade
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genchi, G.; Sinicropi, M.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.; Hu, L.; Zhou, Y.; Min, X.; She, S.; Chen, S.; Huang, M.; et al. Current status, spatial features, health risks, and potential driving factors of soil heavy metal pollution in China at province level. Environ. Pollut. 2020, 266, 114961. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Wang, W.; Li, T.; He, Z.; Yang, X. Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 2019, 651, 3034–3042. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Nordberg, G. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef]
- Yan, L.; Allen, D. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Zhang, D.; Wang, Z.; Wang, L. Quercetin alleviates Cadmium-induced autophagy inhibition via TFEB-dependent lysosomal restoration in primary proximal tubular cells. Ecotoxicol. Environ. Saf. 2021, 208, 111743. [Google Scholar] [CrossRef]
- Yi, L.; Shang, X.J.; Lv, L.; Wang, Y.; Zhang, J.; Quan, C.; Shi, Y.; Liu, Y.; Zhang, L. Cadmium-induced apoptosis of Leydig cells is mediated by excessive mitochondrial fission and inhibition of mitophagy. Cell Death Dis. 2022, 13, 928. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, L.; Liu, Y.; Yu, X.; Qiao, X. Rutin Ameliorates Cadmium-Induced Necroptosis in the Chicken Liver via Inhibiting Oxidative Stress and MAPK/NF-κB Pathway. Biol. Trace Elem. Res. 2022, 200, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Pascal, J. The comings and goings of PARP-1 in response to DNA damage. DNA Repair 2018, 71, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zeng, L.; Zhang, Y.; Wang, M.; Li, Y.; Jia, Y.; Wu, L.; Su, P. Cadmium exposure induces pyroptosis in testicular tissue by increasing oxidative stress and activating the AIM2 inflammasome pathway. Sci. Total Environ. 2022, 847, 157500. [Google Scholar] [CrossRef]
- Luo, T.; Yu, Q.; Zou, H.; Zhao, H.; Gu, J.; Yuan, Y.; Zhu, J.; Bian, J.; Liu, Z. Role of poly (ADP-ribose) polymerase-1 in cadmium-induced cellular DNA damage and cell cycle arrest in rat renal tubular epithelial cell line NRK-52E. Environ. Pollut. 2020, 261, 114149. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Benigni, A. Sirtuins in Renal Health and Disease. J. Am. Soc. Nephrol. 2018, 29, 1799–1809. [Google Scholar] [CrossRef]
- Lin, S.; Xing, H.; Zang, T.; Ruan, X.; Wo, L.; He, M. Sirtuins in mitochondrial stress: Indispensable helpers behind the scenes. Ageing Res. Rev. 2018, 44, 22–32. [Google Scholar] [CrossRef]
- Chen, Q.; Cui, K.; Zhao, Z.; Xu, X.; Liu, Y.; Shen, Y.; Chen, F.; Mai, K.; Ai, Q. LPS stimulation stabilizes HIF-1α by enhancing HIF-1α acetylation via the PARP1-SIRT1 and ACLY-Tip60 pathways in macrophages. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22418. [Google Scholar] [CrossRef]
- Rajamohan, S.; Pillai, V.; Gupta, M.; Sundaresan, N.; Birukov, K.; Samant, S.; Hottiger, M.; Gupta, M. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol. Cell. Biol. 2009, 29, 4116–4129. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, X.; Zhao, R.; Zhang, R.; Xu, C.; Wang, X.; Liu, C.; Hu, X.; Huang, S.; Chen, L. Cadmium results in accumulation of autophagosomes-dependent apoptosis through activating Akt-impaired autophagic flux in neuronal cells. Cell. Signal. 2019, 55, 26–39. [Google Scholar] [CrossRef]
- Kaushal, G.; Shah, S. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef]
- Pi, H.; Li, M.; Zou, L.; Yang, M.; Deng, P.; Fan, T.; Liu, M.; Tian, L.; Tu, M.; Xie, J.; et al. AKT inhibition-mediated dephosphorylation of TFE3 promotes overactive autophagy independent of MTORC1 in cadmium-exposed bone mesenchymal stem cells. Autophagy 2019, 15, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Shi, Y.; Liu, J.; Su, H.; Huang, J.; Zhang, Y.; Peng, C.; Zhou, T.; Sun, Q.; Wan, W.; et al. Acetylation of STX17 (syntaxin 17) controls autophagosome maturation. Autophagy 2020, 17, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Lee, M.; Huang, X.; Messina-Graham, S.; Broxmeyer, H. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells 2014, 32, 1183–1194. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, W.; Zheng, Z.; Wang, W.; Yuan, Y.; Hong, Q.; Lin, J.; Li, X.; Meng, Y. Cigarette smoke-inactivated SIRT1 promotes autophagy-dependent senescence of alveolar epithelial type 2 cells to induce pulmonary fibrosis. Free Radic. Biol. Med. 2021, 166, 116–127. [Google Scholar] [CrossRef]
- Fang, E.; Scheibye-Knudsen, M.; Brace, L.; Kassahun, H.; SenGupta, T.; Nilsen, H.; Mitchell, J.; Croteau, D.; Bohr, V. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 2014, 157, 882–896. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Johansen, T.; Berger, S.; Dou, Z. SIRT1—A new mammalian substrate of nuclear autophagy. Autophagy 2021, 17, 593–595. [Google Scholar] [CrossRef]
- Nabavi, S.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Xu, H.; Yu, W.; Sun, S.; Li, C.; Zhang, Y.; Ren, J. Luteolin Attenuates Doxorubicin-Induced Cardiotoxicity Through Promoting Mitochondrial Autophagy. Front. Physiol. 2020, 11, 113. [Google Scholar] [CrossRef]
- Al-Megrin, W.; Alomar, S.; Alkhuriji, A.; Metwally, D.; Mohamed, S.; Kassab, R.; Abdel Moneim, A.; El-Khadragy, M. Luteolin protects against testicular injury induced by lead acetate by activating the Nrf2/HO-1 pathway. IUBMB Life 2020, 72, 1787–1798. [Google Scholar] [CrossRef]
- Tan, X.; Liu, B.; Lu, J.; Li, S.; Baiyun, R.; Lv, Y.; Lu, Q.; Zhang, Z. Dietary luteolin protects against HgCl-induced renal injury via activation of Nrf2-mediated signaling in rat. J. Inorg. Biochem. 2018, 179, 24–31. [Google Scholar] [CrossRef]
- Domitrović, R.; Cvijanović, O.; Pugel, E.; Zagorac, G.; Mahmutefendić, H.; Škoda, M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology 2013, 310, 115–123. [Google Scholar] [CrossRef]
- Wang, Y.; Su, H.; Song, X.; Fiati Kenston, S.; Zhao, J.; Gu, Y. Luteolin inhibits multi-heavy metal mixture-induced HL7702 cell apoptosis through downregulation of ROS-activated mitochondrial pathway. Int. J. Mol. Med. 2018, 41, 233–241. [Google Scholar] [CrossRef]
- Xu, X.; Yu, Z.; Han, B.; Li, S.; Sun, Y.; Du, Y.; Wang, Z.; Gao, D.; Zhang, Z. Luteolin alleviates inorganic mercury-induced kidney injury via activation of the AMPK/mTOR autophagy pathway. J. Inorg. Biochem. 2021, 224, 111583. [Google Scholar] [CrossRef]
- Li, F.; Wei, R.; Huang, M.; Chen, J.; Li, P.; Ma, Y.; Chen, X. Luteolin can ameliorate renal interstitial fibrosis-induced renal anaemia through the SIRT1/FOXO3 pathway. Food Funct. 2022, 13, 11896–11914. [Google Scholar] [CrossRef]
- Huang, C.; Chen, H.; Lo, C.; Wang, Y.; Li, C.; Liu, K.; Lii, C. Luteolin ameliorates palmitate-induced lipotoxicity in hepatocytes by mediating endoplasmic reticulum stress and autophagy. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2023, 171, 113554. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, C.; Sun, Y.; Zhang, Q.; Lv, M.; Guo, K.; Li, J. Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. Sci. Total Environ. 2019, 689, 1160–1171. [Google Scholar] [CrossRef]
- Shi, Q.; Jin, X.; Fan, R.; Xing, M.; Guo, J.; Zhang, Z.; Zhang, J.; Xu, S. Cadmium-mediated miR-30a-GRP78 leads to JNK-dependent autophagy in chicken kidney. Chemosphere 2019, 215, 710–715. [Google Scholar] [CrossRef]
- Oyagbemi, A.; Adejumobi, O.; Ajibade, T.; Asenuga, E.; Afolabi, J.; Ogunpolu, B.; Falayi, O.; Hassan, F.; Nabofa, E.; Olutayo Omobowale, T.; et al. Luteolin Attenuates Glycerol-Induced Acute Renal Failure and Cardiac Complications through Modulation of Kim-1/NF-κB/Nrf2 Signaling Pathways. J. Diet. Suppl. 2021, 18, 543–565. [Google Scholar] [CrossRef]
- Oyagbemi, A.; Omobowale, T.; Ola-Davies, O.; Asenuga, E.; Ajibade, T.; Adejumobi, O.; Afolabi, J.; Ogunpolu, B.; Falayi, O.; Saba, A.; et al. Luteolin-mediated Kim-1/NF-kB/Nrf2 signaling pathways protects sodium fluoride-induced hypertension and cardiovascular complications. BioFactors 2018, 44, 518–531. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, W.; Li, J.; Gong, Z.; Liu, W.; He, S.; Zou, H.; Song, R.; Liu, G.; Liu, Z. Honokiol Antagonizes Cadmium-Induced Nephrotoxicity in Quail by Alleviating Autophagy Dysfunction, Apoptosis and Mitochondrial UPR Inhibition with Its Antioxidant Properties. Life 2022, 12, 1574. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.; Gobe, G. Kidney Cadmium Toxicity, Diabetes and High Blood Pressure: The Perfect Storm. Tohoku J. Exp. Med. 2017, 241, 65–87. [Google Scholar] [CrossRef]
- Zhuang, J.; Nie, G.; Yang, F.; Dai, X.; Cao, H.; Xing, C.; Hu, G.; Zhang, C. Cadmium induces cytotoxicity through oxidative stress-mediated apoptosis pathway in duck renal tubular epithelial cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2019, 61, 104625. [Google Scholar] [CrossRef]
- Shi, L.; Cao, H.; Luo, J.; Liu, P.; Wang, T.; Hu, G.; Zhang, C. Effects of molybdenum and cadmium on the oxidative damage and kidney apoptosis in Duck. Ecotoxicol. Environ. Saf. 2017, 145, 24–31. [Google Scholar] [CrossRef]
- Liu, L.; Yang, B.; Cheng, Y.; Lin, H. Ameliorative Effects of Selenium on Cadmium-Induced Oxidative Stress and Endoplasmic Reticulum Stress in the Chicken Kidney. Biol. Trace Elem. Res. 2015, 167, 308–319. [Google Scholar] [CrossRef]
- Li, X.; Ge, M.; Zhu, W.; Wang, P.; Wang, J.; Tai, T.; Wang, Y.; Sun, J.; Shi, G. Protective Effects of Astilbin Against Cadmium-Induced Apoptosis in Chicken Kidneys via Endoplasmic Reticulum Stress Signaling Pathway. Biol. Trace Elem. Res. 2022, 200, 4430–4443. [Google Scholar] [CrossRef]
- Hong, X.; Zhao, X.; Wang, G.; Zhang, Z.; Pei, H.; Liu, Z. Luteolin Treatment Protects against Renal Ischemia-Reperfusion Injury in Rats. Mediat. Inflamm. 2017, 2017, 9783893. [Google Scholar] [CrossRef]
- Chu, N.; Zhang, X.; Chen, S.; Zhen, Q.; Wang, Y. Luteolin has a significant protective effect against cadmium-induced injury in lung epithelial Beas-2B cells. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2021, 41, 729–735. [Google Scholar]
- Al-Megrin, W.; Alkhuriji, A.; Yousef, A.; Metwally, D.; Habotta, O.; Kassab, R.; Abdel Moneim, A.; El-Khadragy, M. Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants 2019, 9, 10. [Google Scholar] [CrossRef]
- Owumi, S.; Lewu, D.; Arunsi, U.; Oyelere, A. Luteolin attenuates doxorubicin-induced derangements of liver and kidney by reducing oxidative and inflammatory stress to suppress apoptosis. Hum. Exp. Toxicol. 2021, 40, 1656–1672. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, C.; Li, X.; Zhou, S.; Hua, J.; Huang, J.; Li, Y.; Yang, K.; Zhang, P.; Zhang, Y.; et al. Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1α pathways in NRK-52E rat kidney cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 141, 111436. [Google Scholar] [CrossRef]
- De Leo, E.; Elmonem, M.; Berlingerio, S.; Berquez, M.; Festa, B.; Raso, R.; Bellomo, F.; Starborg, T.; Janssen, M.; Abbaszadeh, Z.; et al. Cell-Based Phenotypic Drug Screening Identifies Luteolin as Candidate Therapeutic for Nephropathic Cystinosis. J. Am. Soc. Nephrol. 2020, 31, 1522–1537. [Google Scholar] [CrossRef]
- Gobe, G.; Crane, D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol. Lett. 2010, 198, 49–55. [Google Scholar] [CrossRef]
- Pavón, N.; Buelna-Chontal, M.; Macías-López, A.; Correa, F.; Uribe-Álvarez, C.; Hernández-Esquivel, L.; Chávez, E. On the oxidative damage by cadmium to kidney mitochondrial functions. Biochem. Cell Biol. = Biochim. Biol. Cell. 2019, 97, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Protective Role of the Essential Trace Elements in the Obviation of Cadmium Toxicity: Glimpses of Mechanisms. Biol. Trace Elem. Res. 2022, 200, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, W.; Zheng, J. Life-cycle exposure to cadmium induced compensatory responses towards oxidative stress in the liver of female zebrafish. Chemosphere 2018, 210, 949–957. [Google Scholar] [CrossRef]
- Taylor, C. Zinc, the pancreas, and diabetes: Insights from rodent studies and future directions. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2005, 18, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, J.; Zhang, K.; Liu, X.; Li, J. Effects of chronic cadmium poisoning on Zn, Cu, Fe, Ca, and metallothionein in liver and kidney of rats. Biol. Trace Elem. Res. 2012, 149, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, K.; Gong, Z.; Luo, T.; Li, J.; Wang, X.; Zou, H.; Song, R.; Zhu, J.; Ma, Y.; et al. N-acetylcysteine delayed cadmium-induced chronic kidney injury by activating the sirtuin 1-P53 signaling pathway. Chem. Biol. Interact. 2023, 369, 110299. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Chu, X.; Guo, H.; Huang, G.; Cui, T.; Huang, B.; Dai, X.; Zhang, C. The activated ATM/AMPK/mTOR axis promotes autophagy in response to oxidative stress-mediated DNA damage co-induced by molybdenum and cadmium in duck testes. Environ. Pollut. 2023, 316, 120574. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, C.; Song, X.; Li, J.; Peng, H.; Qiu, M.; Yang, L.; Du, H.; Jiang, X.; Liu, Y. Long Non-coding RNA Expression Profile in Broiler Liver with Cadmium-Induced Oxidative Damage. Biol. Trace Elem. Res. 2021, 199, 3053–3061. [Google Scholar] [CrossRef]
- Jomova, K.; Hudecova, L.; Lauro, P.; Simunková, M.; Barbierikova, Z.; Malcek, M.; Alwasel, S.; Alhazza, I.; Rhodes, C.; Valko, M. The effect of Luteolin on DNA damage mediated by a copper catalyzed Fenton reaction. J. Inorg. Biochem. 2022, 226, 111635. [Google Scholar] [CrossRef]
- Hurtado-Bagès, S.; Knobloch, G.; Ladurner, A.; Buschbeck, M. The taming of PARP1 and its impact on NAD metabolism. Mol. Metab. 2020, 38, 100950. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Wang, S.; Hou, L.; Cui, Z.; Li, Q.; Huang, H. Selenium alleviates cadmium-induced oxidative stress, endoplasmic reticulum stress and programmed necrosis in chicken testes. Sci. Total Environ. 2023, 863, 160601. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Yuan, Y.; Yu, Q.; Liu, G.; Long, M.; Zhang, K.; Bian, J.; Gu, J.; Zou, H.; Wang, Y.; et al. PARP-1 overexpression contributes to Cadmium-induced death in rat proximal tubular cells via parthanatos and the MAPK signalling pathway. Sci. Rep. 2017, 7, 4331. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Gong, H.; Xi, C.; Fan, W.; Dai, Y.; Zhang, T. Poly(ADP-ribose) polymerase 1 is involved in glucose toxicity through SIRT1 modulation in HepG2 hepatocytes. J. Cell. Biochem. 2011, 112, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.; et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Waldman, M.; Nudelman, V.; Shainberg, A.; Abraham, N.; Kornwoski, R.; Aravot, D.; Arad, M.; Hochhauser, E. PARP-1 inhibition protects the diabetic heart through activation of SIRT1-PGC-1α axis. Exp. Cell Res. 2018, 373, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Pi, H.; Xu, S.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; Li, R.; et al. Melatonin Improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 142, 182–195. [Google Scholar] [CrossRef]
- Hao, R.; Ge, J.; Song, X.; Li, F.; Sun-Waterhouse, D.; Li, D. Cadmium induces ferroptosis and apoptosis by modulating miR-34a-5p/Sirt1axis in PC12 cells. Environ. Toxicol. 2022, 37, 41–51. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Pi, S.; Wei, Z.; Wang, C.; Yang, F.; Li, G.; Nie, G.; Hu, G. Cadmium and molybdenum co-exposure triggers autophagy via CYP450s/ROS pathway in duck renal tubular epithelial cells. Sci. Total Environ. 2021, 759, 143570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Z.; Hu, R.; Pi, S.; Wei, Z.; Wang, C.; Yang, F.; Xing, C.; Nie, G.; Hu, G. New insights into crosstalk between pyroptosis and autophagy co-induced by molybdenum and cadmium in duck renal tubular epithelial cells. J. Hazard. Mater. 2021, 416, 126138. [Google Scholar] [CrossRef]
- Sun, J.; Bian, Y.; Ma, Y.; Ali, W.; Wang, T.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z.; Zou, H. Melatonin alleviates cadmium-induced nonalcoholic fatty liver disease in ducks by alleviating autophagic flow arrest via PPAR-α and reducing oxidative stress. Poult. Sci. 2023, 102, 102835. [Google Scholar] [CrossRef]
- Parzych, K.; Klionsky, D. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pi, H.; Yang, Z.; Reiter, R.; Xu, S.; Chen, X.; Chen, C.; Zhang, L.; Yang, M.; Li, Y.; et al. Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J. Pineal Res. 2016, 61, 353–369. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, H.; Wang, M.G.; Yang, D.B.; Wang, Z.Y.; Wang, L. Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. Cell Death Dis. 2017, 8, e3099. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, Y.; Fan, X.; Liu, X.; Peng, L.; Ci, X. Oridonin Sensitizes Cisplatin-Induced Apoptosis via AMPK/Akt/mTOR-Dependent Autophagosome Accumulation in A549 Cells. Front. Oncol. 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- El-Kott, A.; Abd-Lateif, A.; Khalifa, H.; Morsy, K.; Ibrahim, E.; Bin-Jumah, M.; Abdel-Daim, M.; Aleya, L. Kaempferol protects against cadmium chloride-induced hippocampal damage and memory deficits by activation of silent information regulator 1 and inhibition of poly (ADP-Ribose) polymerase-1. Sci. Total Environ. 2020, 728, 138832. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 18424. [Google Scholar] [CrossRef]
- Song, Y.; Lee, Y.; Kim, J.; Ham, D.; Kang, E.; Cha, B.; Lee, H.; Lee, B. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy 2015, 11, 46–59. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Li, J.; Dong, W.; Huang, Q.; Wang, X.; Deng, K.; Ali, W.; Song, R.; Zou, H.; Ran, D.; et al. Luteolin Alleviates Cadmium-Induced Kidney Injury by Inhibiting Oxidative DNA Damage and Repairing Autophagic Flux Blockade in Chickens. Antioxidants 2024, 13, 525. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050525
Zhang K, Li J, Dong W, Huang Q, Wang X, Deng K, Ali W, Song R, Zou H, Ran D, et al. Luteolin Alleviates Cadmium-Induced Kidney Injury by Inhibiting Oxidative DNA Damage and Repairing Autophagic Flux Blockade in Chickens. Antioxidants. 2024; 13(5):525. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050525
Chicago/Turabian StyleZhang, Kanglei, Jiahui Li, Wenxuan Dong, Qing Huang, Xueru Wang, Kai Deng, Waseem Ali, Ruilong Song, Hui Zou, Di Ran, and et al. 2024. "Luteolin Alleviates Cadmium-Induced Kidney Injury by Inhibiting Oxidative DNA Damage and Repairing Autophagic Flux Blockade in Chickens" Antioxidants 13, no. 5: 525. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050525