Inhibition of Lipid Accumulation and Oxidation in Hepatocytes by Bioactive Bean Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Evaluation of Cell Viability and Lipid Droplet Accumulation Inhibition Assay
2.3. Analysis of TAG and TGOOH Species
2.4. Metabolites Profiles
3. Results and Discussion
3.1. Evaluation of Cell Viability under Co-Treatment with Fatty Acids and Bean Extracts
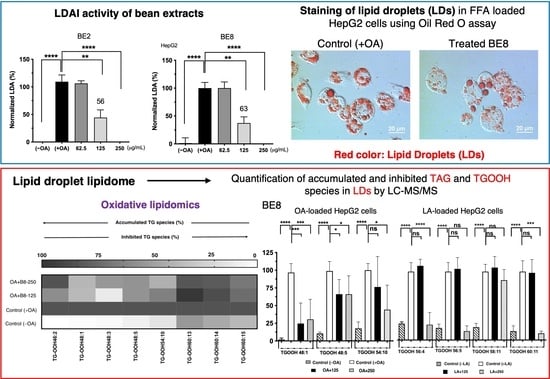
3.2. Lipid Droplet Accumulation Inhibition Activity of Selected Bean Extracts
3.3. Quantification of the Effect on Inhibiting the Accumulation of TAG and TGOOH Species
3.3.1. Analysis of Accumulated TAG and TGOOH Species Induced by Oleic Acid in Hepatocytes
3.3.2. Quantification of TAG and TGOOH Species Induced by Linoleic Acid
From Traditional and Imaging Approaches to Advanced MS Approaches
MS/MS-Based Detection of Oxidized Molecular Lipid Species
3.4. Metabolite Profiling of Bioactive Extracts of Beans
3.5. Strengths, Limitations and Future Prospects of the Oxidative Lipidomic Approach Based on the LDAI Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Müller, F.A.; Sturla, S.J. Human in vitro models of nonalcoholic fatty liver disease. Curr. Opin. Toxicol. 2019, 16, 9–16. [Google Scholar] [CrossRef]
- Shao, G.; Lui, Y.; Lu, L.; Zhang, G.; Zhou, W.; Wu, T.; Wang, L.; Xu, H.; Ji, G. The Pathogenesis of HCC Driven by NASH and the Preventive and Therapeutic Effects of Natural Products. Front. Pharmacol. 2022, 13, 9440088. [Google Scholar] [CrossRef] [PubMed]
- Ziamajidi, N.; Khaghani, S.; Hassanzadeh, G.; Vardasbi, S.; Ahmadian, S.; Nowrouzi, A.; Ghaffari, S.M.; Abdirad, A. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARα and SREBP-1. Food Chem. Toxicol. 2013, 58, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-Alcoholic Fatty Liver Disease—A Global Public Health Perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, S.; DeLeeuw, P.; Satapathy, S.K. A Review of Current and Upcoming Treatment Modalities in Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Hepatic Med. Evid. Res. 2019, 11, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, M.; Neuschwander-Tetri, B.A. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterol. Hepatol. 2006, 2, 282–291. [Google Scholar]
- Wang, T.; Zhao, J.; Li, X.; Xu, Q.; Liu, Y.; Khan, I.A.; Yang, S. New Alkaloids from Green Vegetable Soybeans and Their Inhibitory Activities on the Proliferation of Concanavalin A-Activated Lymphocytes. J. Agric. Food Chem. 2016, 64, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Dolgova, N.; Uhlemann, E.-M.E.; Boniecki, M.T.; Vizeacoumar, F.S.; Ralle, M.; Tonelli, M.; Abbas, S.A.; Patry, J.; Elhasasna, H.; Freywald, A.; et al. MEMO1 is a Metal Containing Regulator of Iron Homeostasis in Cancer Cells. bioRxiv 2023. [Google Scholar] [CrossRef]
- Walther, T.C.; Chung, J.; Farese, R.V., Jr. Lipid Droplet Biogenesis. Annu. Rev. Cell Dev. Biol. 2017, 33, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.L.S.; Barreto, E.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.W.; Miyoshi, H.; Mashek, D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011, 121, 2102–2110. [Google Scholar] [CrossRef]
- Dibwe, D.F.; Oba, S.; Takeishi, N.; Sakurai, T.; Tsukui, T.; Chiba, H.; Hui, S.P. Food-Derived β-Carboline Alkaloids Ameliorate Lipid Droplet Accumulation in Human Hepatocytes. Pharmaceuticals 2022, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V. Lipid Droplets and Liver Disease: From Basic Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Krahmer, N.; Farese, R.V.; Walther, T.C. Balancing the Fat: Lipid Droplets and Human Disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Hauck, A.K.; Bernlohr, D.A. Oxidative stress and lipotoxicity. J. Lipid Res. 2016, 57, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Mashek, D.G. Hepatic Lipid Droplets: A Balancing Act between Energy Storage and Metabolic Dysfunction in NAFLD. Mol. Metab. 2021, 50, 101115. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.R.d.A.; Diniz, M.F.F.M.; de Medeiros-Filho, J.E.M.; de Araújo, M.S.T. Metabolic Syndrome and Risk Factors for Non-Alcoholic Fatty Liver Disease. Arq. Gastroenterol. 2012, 49, 89–96. [Google Scholar] [CrossRef]
- López Panqueva, R.d.P. Pathological Aspects of Fatty Liver Disease. Rev. Colomb. Gastroenterol. 2014, 29, 82–88. [Google Scholar]
- Hernández-Pérez, E.; León García, P.E.; López-Díazguerrero Henne, W.M.; Reese, M.L.; Goodman, J.M. The Assembly of Lipid Droplets and Their Roles in Challenged Cells. EMBO J. 2018, 37, e98947. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, E.; León García, P.E.; López-Díazguerrero, N.E.; Rivera-Cabrera, F.; Del Ángel Benítez, E. Liver steatosis and nonalcoholic steatohepatitis: From pathogenesis to therapy. Medwave 2016, 16, e6535. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Okanoue, T.; Nakajima, A. Phase 3 Drug Pipelines in the Treatment of Non-Alcoholic Steatohepatitis. Hepatol. Res. 2019, 49, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Zhang, Q.; Du, Z.; Gai, Z.; Wu, J.; et al. An Overview of Lipid Metabolism and Nonalcoholic Fatty Liver Disease. Biomed Res. Int. 2020, 2020, 4020249. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.C. Molecular Breeding and Nutritional Aspects of Buckwheat; Elsevier: Amsterdam, The Netherlands, 2016; pp. 109–129. [Google Scholar]
- Cheng, C.; Li, Z.; Zhao, X.; Liao, C.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X. Natural alkaloid and polyphenol compounds targeting lipid metabolism: Treatment implications in metabolic diseases. Eur. J. Pharmacol. 2020, 870, 172922. [Google Scholar] [CrossRef]
- Dibwe, D.F.; Takeishi, N.; Oba, S.; Sakurai, A.; Sakurai, T.; Tsukui, T.; Chiba, H.; Hui, S.P. Identification of a b-carboline alkaloid from chemoselectively derived vanilla bean extract and its prevention of lipid droplet accumulation in human hepatocytes (HepG2). Molecules 2023, 15, 8024. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhuo, S.; Zhang, B.; Zhao, X.; Liu, Y.; Liao, C.; Quan, J.; Li, Z.; Bode, A.M.; Cao, Y.; et al. Treatment implications of natural compounds targeting lipid metabolism in nonalcoholic fatty liver disease, obesity and cancer. Int. J. Biol. Sci. 2019, 15, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Busconi, M.; Lucini, L.; Soffritti, G.; Bernardi, J.; Bernardo, L.; Brunschwig, C.; Lepers-Andrzejewski, S.; Raharivelomanana, P.; Fernandez, J.A. Phenolic Profiling for Traceability of Vanilla × tahitensis. Front. Plant Sci. 2017, 8, 1746. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Chen, Z.; Fuda, H.; Furukawa, T.; Oura, K.; Sakurai, T.; Hui, S.P.; Chiba, H. Novel Fluorescence-Based Method to Characterize the Antioxidative Effects of Food Metabolites on Lipid Droplets in Cultured Hepatocytes. J. Agric. Food Chem. 2019, 67, 9934–9941. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, X.; Chiba, H.; Hui, S.-P. Investigating oxidized lipids in an omics way: Oxidative lipidomics in biological applications using mass spectrometry. Med. Mass Spectrom. 2022, 6, 72–84. [Google Scholar] [CrossRef]
- Sazaki, I.; Sakurai, T.; Yamahata, A.; Mogi, S.; Inoue, N.; Ishida, K.; Kikkai, A.; Takeshita, H.; Sakurai, A.; Takahashi, Y.; et al. Oxidized Low-Density Lipoproteins Trigger Hepatocellular Oxidative Stress with the Formation of Cholesteryl Ester Hydroperoxide-Enriched Lipid Droplets. Int. J. Mol. Sci. 2023, 24, 4281. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Z.; Fuda, H.; Tsukui, T.; Wu, X.; Shen, N.; Saito, N.; Chiba, H.; Hui, S.-P. Oxidative Stress Linked Organ Lipid Hydroperoxidation and Dysregulation in Mouse Model of Nonalcoholic Steatohepatitis: Revealed by Lipidomic Profiling of Liver and Kidney. Antioxidants 2021, 10, 1602. [Google Scholar] [CrossRef] [PubMed]





| Inhibited TAG Species | ||||
|---|---|---|---|---|
| Range of Inhibition (%) | OA | LA | ||
| BE2 | BE8 | BE2 | BE8 | |
| 0–49 | 1 | 14 | 0 | 1 |
| 50–85 | 8 | 4 | 1 | 14 |
| Inhibited TGOOH Species | ||||
|---|---|---|---|---|
| Range of Inhibition (%) | OA | LA | ||
| BE2 | BE8 | BE2 | BE8 | |
| 0–49 | 1 | 2 | 0 | 3 |
| 50–85 | 1 | 1 | 0 | 2 |
| Compounds | RT | Ion | Calc. m/z | Exptl. m/z | ppm |
|---|---|---|---|---|---|
| 1. Gallic acid hydrate | 1.83 | [M + H]+ | 171.0288 | 171.0289 | 0.58 |
| 2. Syringic acid | 2.16 | [M + H]+ | 198.0528 | 198.0524 | −2.02 |
| 3. 4-Hydroxybenzoic acid | 2.1 | [M + H]+ | 139.0390 | 139.0382 | −5.75 |
| 4. Vanillic acid | 2.02 | [M + H]+ | 169.0495 | 169.0491 | −2.37 |
| 5. 3,4-Dihydroxybenzoic aldehyde | 2.1 | [M + H]+ | 139.0390 | 139.0382 | −5.75 |
| 6. 4-Hydroxybenzoic aldehyde | 3.13 | [M + H]+ | 123.0441 | 123.0439 | −1.63 |
| 7. Vanillin | 3.13 | [M + H]+ | 153.0546 | 153.0545 | −0.65 |
| 8. Daidzein | 2.75 | [M + H]+ | 255.0642 | 255.0650 | 3.14 |
| 9. Genistein | 2.96 | [M + H]+ | 271.0601 | 271.0599 | −0.74 |
| 10. Anthocyanin derivative | 2.24 | [M + H]+ | 291.0863 | 291.0851 | −4.12 |
| 11. Genistein glucoside | 2.69 | [M + H]+ | 431.0984 | 431.0991 | 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibwe, D.F.; Kitayama, E.; Oba, S.; Takeishi, N.; Chiba, H.; Hui, S.-P. Inhibition of Lipid Accumulation and Oxidation in Hepatocytes by Bioactive Bean Extracts. Antioxidants 2024, 13, 513. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050513
Dibwe DF, Kitayama E, Oba S, Takeishi N, Chiba H, Hui S-P. Inhibition of Lipid Accumulation and Oxidation in Hepatocytes by Bioactive Bean Extracts. Antioxidants. 2024; 13(5):513. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050513
Chicago/Turabian StyleDibwe, Dya Fita, Emi Kitayama, Saki Oba, Nire Takeishi, Hitoshi Chiba, and Shu-Ping Hui. 2024. "Inhibition of Lipid Accumulation and Oxidation in Hepatocytes by Bioactive Bean Extracts" Antioxidants 13, no. 5: 513. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13050513