Dynamic Climate Influence on Magnesium Isotope Variation in Saline Lacustrine Dolomite: A Case Study of the Qianjiang Formation, Jianghan Basin
Abstract
:1. Introduction
2. Geological Settings

3. Materials and Methods
3.1. Elemental Composition Analyses
3.2. Carbon and Oxygen Isotopes
3.3. Purification and Testing of Mg Isotopes
4. Results
4.1. Petrological Characteristics
4.2. Geochemical Characteristics
4.2.1. Elemental Geochemistry
4.2.2. Stable Isotopes
5. Discussion
5.1. Paleoclimate and Basin Provenance
5.1.1. Assessing the Impact of Diagenesis
5.1.2. Paleoclimate Characteristics
5.1.3. Basin Provenance

5.2. Dolomite Deposition Pattern in Saline Lake
5.2.1. δ26Mg of the River
5.2.2. Numerical Model of Magnesium Cycling and Isotopic Mass Balance in Saline Lake
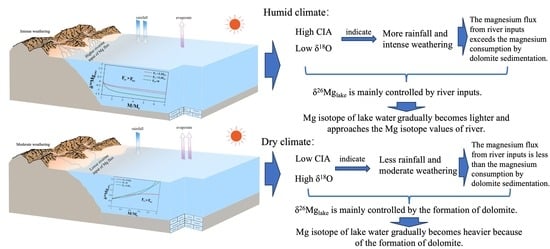
5.2.3. Dolomite Magnesium Isotope System Controlled by Riverine Input under Humid Climate Conditions
5.2.4. Dolomite Magnesium Isotope System Controlled by Dolomite Precipitation under Relative Dry Climate Conditions
5.2.5. Magnesium Isotope Variations in Dolomite at the Centimeter Sedimentary Scale Provide Support for Macroscopic Sedimentary Scale Models
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Land, L.S. Failure to Precipitate Dolomite at 25 °C from Dilute Solution Despite 1000-Fold Oversaturation after 32 Years. Aquat. Geochem. 1998, 4, 361–368. [Google Scholar] [CrossRef]
- Sibley, D. Dolomites: A Volume in Honour of Dolomieu. Bruce Purser, Maurice Tucker, Donald Zenger. J. Geol. 1995, 103, 729. [Google Scholar] [CrossRef]
- Machel, H.G. Concepts and models of dolomitization; a critical reappraisal. Geol. Soc. Spec. Publ. 2004, 235, 7–63. [Google Scholar] [CrossRef]
- Braithwaite, C.J.R.; Rizzi, G.; Darke, G. The Geometry and Petrogenesis of Dolomite Hydrocarbon Reservoirs. Geol. Soc. Lond. 2004, 235, 1–6. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, D.; Jin, Z.; Liu, C.; Zhang, D.; He, Z. Coupled alteration of hydrothermal fluids and thermal sulfate reduction (TSR) in ancient dolomite reservoirs—An example from Sinian Dengying Formation in Sichuan Basin, Southern China. Precambrian Res. 2016, 285, 39–57. [Google Scholar] [CrossRef]
- Johnson, C.; Beard, B.L.; Albarède, F. Geochemistry of Non-Traditional Stable Isotopes; Reviews in Mineralogy & Geochemistry Series; De Gruyter: Berlin, Germany, 2004; Volume 55. [Google Scholar] [CrossRef]
- Geske, A.; Goldstein, R.H.; Mavromatis, V.; Richter, D.K.; Buhl, D.; Kluge, T.; John, C.M.; Immenhauser, A. The magnesium isotope (δ26Mg) signature of dolomites. Geochim. Cosmochim. Acta 2015, 149, 131–151. [Google Scholar] [CrossRef]
- Teng, F.-Z. Magnesium isotope Geochemistry. Rev. Mineral. Geochem. 2017, 82, 219–287. [Google Scholar] [CrossRef]
- Li, J.; Hao, C.; Wang, Z.; Dong, L.; Wang, Y.; Huang, K.-J.; Lang, X.; Huang, T.; Yuan, H.; Zhou, C.; et al. Continental weathering intensity during the termination of the Marinoan Snowball Earth: Mg isotope evidence from the basal Doushantuo cap carbonate in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 552, 109774. [Google Scholar] [CrossRef]
- Teng, F.-Z.; Li, W.-Y.; Rudnick, R.L.; Gardner, L.R. Contrasting lithium and magnesium isotope fractionation during continental weathering. Earth Planet. Sci. Lett. 2010, 300, 63–71. [Google Scholar] [CrossRef]
- Galy, A.; Bar-Matthews, M.; Halicz, L.; O’Nions, R.K. Mg isotopic composition of carbonate: Insight from speleothem formation. Earth Planet. Sci. Lett. 2002, 201, 105–115. [Google Scholar] [CrossRef]
- Wimpenny, J.; Burton, K.W.; James, R.H.; Gannoun, A.; Mokadem, F.; Gislason, S.R. The behaviour of magnesium and its isotopes during glacial weathering in an ancient shield terrain in West Greenland. Earth Planet. Sci. Lett. 2011, 304, 260–269. [Google Scholar] [CrossRef]
- Fantle, M.S.; Higgins, J. The effects of diagenesis and dolomitization on Ca and Mg isotopes in marine platform carbonates: Implications for the geochemical cycles of Ca and Mg. Geochim. Cosmochim. Acta 2014, 142, 458–481. [Google Scholar] [CrossRef]
- Wimpenny, J.; Colla, C.A.; Yin, Q.Z.; Rustad, J.R.; Casey, W.H. Investigating the behaviour of Mg isotopes during the formation of clay minerals. Geochim. Cosmochim. Acta 2014, 128, 178–194. [Google Scholar] [CrossRef]
- Huang, K.-J.; Shen, B.; Xianguo, L.; Tang, W.-B.; Yang, P.; Shan, K.; Kaufman, A.J.; Haoran, M.; Fangbing, L. Magnesium isotopic compositions of the Mesoproterozoic dolostones: Implications for Mg isotopic systematics of marine carbonates. Geochim. Cosmochim. Acta 2015, 164, 333–351. [Google Scholar] [CrossRef]
- Peng, Y.; Shen, B.; Lang, X.-G.; Huang, K.-J.; Chen, J.-T.; Yan, Z.; Tang, W.-B.; Ke, S.; Ma, H.-R.; Li, F.-B. Constraining dolomitization by Mg isotopes: A case study from partially dolomitized limestones of the middle Cambrian Xuzhuang Formation, North China. Geochem. Geophys. Geosystems 2016, 17, 1109–1129. [Google Scholar] [CrossRef]
- Ning, M.; Lang, X.; Huang, K.; Li, C.; Huang, T.; Yuan, H.; Xing, C.; Yang, R.; Shen, B. Towards understanding the origin of massive dolostones. Earth Planet. Sci. Lett. 2020, 545, 116403. [Google Scholar] [CrossRef]
- Labuhn, I.; Tell, F.; von Grafenstein, U.; Hammarlund, D.; Kuhnert, H.; Minster, B. A modern snapshot of the isotopic composition of lacustrine biogenic carbonates—Records of seasonal water temperature variability. Biogeosciences 2022, 19, 2759–2777. [Google Scholar] [CrossRef]
- Martin, J.B. Carbonate minerals in the global carbon cycle. Chem. Geol. 2017, 449, 58–72. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Fang, Y.; Ma, J.; Shen, B.; Huang, F.; Li, L.; Ning, M.; Zhai, L.; Zhang, W. Mg, C and O isotopic compositions of Late Cretaceous lacustrine dolomite and travertine in the northern Tianshan Mountains, Northwest China. Chem. Geol. 2020, 541, 119569. [Google Scholar] [CrossRef]
- Guo, P.; Wen, H.; Li, C.; He, H.; Sánchez-Román, M. Lacustrine dolomite in deep time: What really matters in early dolomite formation and accumulation? Earth-Sci. Rev. 2023, 246, 104575. [Google Scholar] [CrossRef]
- Wei, R.; Ma, H.; Jin, Z.; Wang, T.; Zhang, C.; Wang, Y.; Dong, L. New insights into the carbon cycle and depositional models of the Eocene saline lake, Jianghan basin, China. Mar. Pet. Geol. 2023, 149, 106079. [Google Scholar] [CrossRef]
- Xia, Z.; Lin, Y.; Wei, H.; Hu, Z.; Liu, C.; Li, W. Reconstruct hydrological history of terrestrial saline lakes using Mg isotopes in halite: A case study of the Quaternary Dalangtan playa in Qaidam Basin, NW China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 587, 110804. [Google Scholar] [CrossRef]
- Ning, M.; Wang, Y.; McKenzie, J.A.; Vasconcelos, C.; Li, C.; Shen, A.; Liang, F.; Shen, B. Dolomite formation during penecontemporaneous subaerial diagenesis: Evidence from modern dolomite crusts forming in lagoon Brejo do Espinho, Brazil. J. Geol. Soc. 2024, 181, 159. [Google Scholar] [CrossRef]
- Shalev, N.; Bontognali, T.R.R.; Vance, D. Sabkha dolomite as an archive for the magnesium isotope composition of seawater. Geology 2020, 49, 253–257. [Google Scholar] [CrossRef]
- Arribas, M.E.; Bustillo, A.; Tsige, M. Lacustrine chalky carbonates: Origin, physical properties and diagenesis (Palaeogene of the Madrid Basin, Spain). Sediment. Geol. 2004, 166, 335–351. [Google Scholar] [CrossRef]
- Boak, J.; Poole, S. Mineralogy of the Green River Formation in the Piceance Creek Basin, Colorado. In Stratigraphy and Paleolimnology of the Green River Formation, Western USA; Springer: Dordrecht, The Netherlands, 2015; Volume 1, pp. 183–209. [Google Scholar]
- Bustillo, M.A.; Arribas, M.E.; Bustillo, M. Dolomitization and silicification in low-energy lacustrine carbonates (Paleogene, Madrid Basin, Spain). Sediment. Geol. 2002, 151, 107–126. [Google Scholar] [CrossRef]
- Calvo, J.P.; Jones, B.F.; Bustillo, M.; Fort, R.; Alonso Zarza, A.M.; Kendall, C. Sedimentology and geochemistry of carbonates from lacustrine sequences in the Madrid Basin, central Spain. Chem. Geol. 1995, 123, 173–191. [Google Scholar] [CrossRef]
- Casado, A.I.; Alonso-Zarza, A.M.; La Iglesia, Á. Morphology and origin of dolomite in paleosols and lacustrine sequences. Examples from the Miocene of the Madrid Basin. Sediment. Geol. 2014, 312, 50–62. [Google Scholar] [CrossRef]
- Donnelly, T.H.; Jackson, M.J. Sedimentology and geochemistry of a mid-Proterozoic lacustrine unit from northern Australia. Sediment. Geol. 1988, 58, 145–169. [Google Scholar] [CrossRef]
- García Del Cura, M.A.; Calvo, J.P.; Ordóñez, S.; Jones, B.F.; Cañaveras, J.C. Petrographic and geochemical evidence for the formation of primary, bacterially induced lacustrine dolomite: La Roda ‘white earth’ (Pliocene, central Spain). Sedimentology 2001, 48, 897–915. [Google Scholar] [CrossRef]
- Janaway, T.M.; Parnell, J. Carbonate production within the orcadian basin, Northern Scotland: A petrographic and geochemical study. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1989, 70, 89–105. [Google Scholar] [CrossRef]
- Murphy, J.T.; Lowenstein, T.K.; Pietras, J.T. Preservation of primary lake signatures in alkaline earth carbonates of the Eocene Green River Wilkins Peak-Laney Member transition zone. Sediment. Geol. 2014, 314, 75–91. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; McKenzie, J.A.; de Luca Rebello Wagener, A.; Rivadeneyra, M.A.; Vasconcelos, C. Presence of sulfate does not inhibit low-temperature dolomite precipitation. Earth Planet. Sci. Lett. 2009, 285, 131–139. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Vasconcelos, C.; Schmid, T.; Dittrich, M.; McKenzie, J.A.; Zenobi, R.; Rivadeneyra, M.A. Aerobic microbial dolomite at the nanometer scale: Implications for the geologic record. Geology 2008, 36, 879–882. [Google Scholar] [CrossRef]
- Shaked Gelband, D.; Edelman-Furstenberg, Y.; Stein, M.; Starinsky, A. Formation of lacustrine dolomite in the late Miocene marginal lakes of the East Mediterranean (Northern Israel). Sedimentology 2019, 66, 2950–2975. [Google Scholar] [CrossRef]
- Valero Garcés, B.L. Lacustrine deposition and related volcanism in a transtensional tectonic setting: Upper Stephanian-Lower Autunian in the Aragón-Béarn Basin, western Pyrenees (Spain-France). Sediment. Geol. 1993, 83, 133–160. [Google Scholar] [CrossRef]
- Wanas, H.A.; Sallam, E. Abiotically-formed, primary dolomite in the mid-Eocene lacustrine succession at Gebel El-Goza El-Hamra, NE Egypt: An approach to the role of smectitic clays. Sediment. Geol. 2016, 343, 132–140. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, X.-W.; Wan, L.; Ke, S.; Liu, S.-A.; Han, G.; Guo, H.; Dong, A. Fractionation of Mg isotopes by clay formation and calcite precipitation in groundwater with long residence times in a sandstone aquifer, Ordos Basin, China. Geochim. Cosmochim. Acta 2018, 237, 261–274. [Google Scholar] [CrossRef]
- Tipper, E.T.; Galy, A.; Gaillardet, J.; Bickle, M.J.; Elderfield, H.; Carder, E.A. The magnesium isotope budget of the modern ocean: Constraints from riverine magnesium isotope ratios. Earth Planet. Sci. Lett. 2006, 250, 241–253. [Google Scholar] [CrossRef]
- Herlinger, R., Jr.; Zambonato, E.E.; De Ros, L.F. Influence of Diagenesis On the Quality of Lower Cretaceous Pre-salt Lacustrine Carbonate Reservoirs from Northern Campos Basin, Offshore Brazil. J. Sediment. Res. 2017, 87, 1285–1313. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Cao, Z.; Tao, S.; Felix, M.; Kong, Y.; Zhang, Y. Characteristics and formation mechanism of multi-source mixed sedimentary rocks in a saline lake, a case study of the Permian Lucaogou Formation in the Jimusaer Sag, northwest China. Mar. Pet. Geol. 2019, 102, 704–724. [Google Scholar] [CrossRef]
- Wu, L.; Mei, L.; Paton, D.A.; Guo, P.; Liu, Y.; Luo, J.; Wang, D.; Li, M.; Zhang, P.; Wen, H. Deciphering the origin of the Cenozoic intracontinental rifting and volcanism in eastern China using integrated evidence from the Jianghan Basin. Gondwana Res. 2018, 64, 67–83. [Google Scholar] [CrossRef]
- Kong, X.; Jiang, Z.; Ju, B.; Liang, C.; Cai, Y.; Wu, S. Fine-grained carbonate formation and organic matter enrichment in an Eocene saline rift lake (Qianjiang Depression): Constraints from depositional environment and material source. Mar. Pet. Geol. 2022, 138, 105534. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Qian, M.; Ma, X.; Jiang, Q.; Li, Z.; Tao, G.; Wu, S. What are in pyrolysis S1 peak and what are missed? Petroleum compositional characteristics revealed from programed pyrolysis and implications for shale oil mobility and resource potential. Int. J. Coal Geol. 2020, 217, 103321. [Google Scholar] [CrossRef]
- Huang, C.; Hinnov, L. Evolution of an Eocene–Oligocene saline lake depositional system and its controlling factors, Jianghan Basin, China. J. Earth Sci. 2014, 25, 959–976. [Google Scholar] [CrossRef]
- Fang, Q.; Li, Y. Exhaustive brine production and complete CO₂ storage in Jianghan Basin of China. Environ. Earth Sci. 2014, 72, 1541–1553. [Google Scholar] [CrossRef]
- Xu, L.; Yan, C.; Yu, H.; Wang, B.; Yu, F.; Wang, D. Chronology of Paleogene volcanic rocks in Jianghan Basin. Oil Gas Geol. 1995, 16, 132–137. [Google Scholar]
- Nie, X.; Lu, J.; Djaroun, R.R.; Wang, P.; Li, J.; Zhang, C. Oil content prediction of lacustrine organic-rich shale from wireline logs: A case study of intersalt reservoirs in the Qianjiang Sag, Jianghan Basin, China. Interpretation 2020, 8, SL79–SL88. [Google Scholar] [CrossRef]
- Accordi, G.; Carbone, F. Evolution of the siliciclastic-carbonate shelf system of the northern Kenyan coastal belt in response to Late Pleistocene-Holocene relative sea level changes. J. Afr. Earth Sci. 2016, 123, 234–257. [Google Scholar] [CrossRef]
- Shen, J.J.; Chen, K.Q.; Liu, Y.; Chen, F.F.; Qiu, X.S.; Ma, X.Q.; Meng, J.H. Sedimentary facies of Paleogene lacustrine dolomicrite and implications for petroleum reservoirs in the southern Qianjiang Depression, China. Open Geosci. 2019, 11, 513–532. [Google Scholar] [CrossRef]
- Galy, A.; Yoffe, O.; Janney, P.E.; Williams, R.W.; Cloquet, C.; Alard, O.; Halicz, L.; Wadhwa, M.; Hutcheon, I.D.; Ramon, E.; et al. Magnesium isotope heterogeneity of the isotopic standard SRM980 and new reference materials for magnesium-isotope-ratio measurements. J. Anal. At. Spectrom. 2003, 18, 1352–1356. [Google Scholar] [CrossRef]
- Bao, Z.; Huang, K.; Huang, T.; Shen, B.; Zong, C.; Chen, K.; Yuan, H. Precise magnesium isotope analyses of high-K and low-Mg rocks by MC-ICP-MS. J. Anal. At. Spectrom. 2019, 34, 940–953. [Google Scholar] [CrossRef]
- Jacobsen, S.B.; Kaufman, A.J. The Sr, C and O isotopic evolution of Neoproterozoic seawater. Chem. Geol. 1999, 161, 37–57. [Google Scholar] [CrossRef]
- Knauth, L.P.; Kennedy, M.J. The late Precambrian greening of the Earth. Nature 2009, 460, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Gómez Peral, L.E.; Poiré, D.G.; Strauss, H.; Zimmermann, U. Chemostratigraphy and diagenetic constraints on Neoproterozoic carbonate successions from the Sierras Bayas Group, Tandilia System, Argentina. Chem. Geol. 2007, 237, 109–128. [Google Scholar] [CrossRef]
- Brand, U.; Veizer, J. Chemical Diagenesis of a Multicomponent Carbonate System;1, Trace-Elements. J. Sediment. Res. 1980, 50, 1219–1236. [Google Scholar] [CrossRef]
- Moradi, A.V.; Sari, A.; Akkaya, P. Geochemistry of the Miocene oil shale (Hancili Formation) in the Cankiri-Corum Basin, Central Turkey: Implications for Paleoclimate conditions, source-area weathering, provenance and tectonic setting. Sediment. Geol. 2016, 341, 289–303. [Google Scholar] [CrossRef]
- Critelli, S.; Mongelli, G.; Perri, F.; Martín-Algarra, A.; Martín-Martín, M.; Perrone, V.; Dominici, R.; Sonnino, M.; Zaghloul, M.N. Compositional and Geochemical Signatures for the Sedimentary Evolution of the Middle Triassic-Lower Jurassic Continental Redbeds from Western-Central Mediterranean Alpine Chains. J. Geol. 2008, 116, 375–386. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Fedo, C.M.; Nesbitt, H.W.; Young, G.M. Unraveling the effects of potassium metasomatism in sedimentary rocks and paleosols, with implications for paleoweathering conditions and provenance. Geology 1995, 23, 921–924. [Google Scholar] [CrossRef]
- Hillman, A.L.; O’Quinn, R.F.; Abbott, M.B.; Bain, D.J. A Holocene history of the Indian monsoon from Qilu Lake, southwestern China. Quat. Sci. Rev. 2020, 227, 106051. [Google Scholar] [CrossRef]
- Dai, S. Petroleum Geology of Jianghan Saline Basin; Petroleum Industry Publishing House: Beijing, China, 1997. [Google Scholar]
- Taylor, S.G.; Bradley, C.E. Optimal Ordering Strategies for Announced Price Increases. Oper. Res. 1985, 33, 237–468. [Google Scholar] [CrossRef]
- Bhatia, M.R.; Crook, K.A.W. Trace element characteristics of graywackes and tectonic setting discrimination of sedimentary basins. Contrib. Mineral. Petrol. 1986, 92, 181–193. [Google Scholar] [CrossRef]
- Cullers, R.L.; Podkovyrov, V.N. Geochemistry of the Mesoproterozoic Lakhanda shales in southeastern Yakutia, Russia: Implications for mineralogical and provenance control, and recycling. Precambrian Res. 2000, 104, 77–93. [Google Scholar] [CrossRef]
- Floyd, P.A.; Leveridge, B.E. Tectonic environment of the Devonian Gramscatho basin, south Cornwall: Framework mode and geochemical evidence from turbiditic sandstones. J. Geol. Soc. 1987, 144, 531–542. [Google Scholar] [CrossRef]
- Cullers, R.L. Implications of elemental concentrations for provenance, redox conditions, and metamorphic studies of shales and limestones near Pueblo, CO, USA. Chem. Geol. 2002, 191, 305–327. [Google Scholar] [CrossRef]
- Li, W.; Bialik, O.M.; Wang, X.; Yang, T.; Hu, Z.; Huang, Q.; Zhao, S.; Waldmann, N.D. Effects of early diagenesis on Mg isotopes in dolomite: The roles of Mn(IV)-reduction and recrystallization. Geochim. Cosmochim. Acta 2019, 250, 1–17. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Penman, D.E.; Caves Rugenstein, J.K.; Ibarra, D.E.; Winnick, M.J. Silicate weathering as a feedback and forcing in Earth’s climate and carbon cycle. Earth-Sci. Rev. 2020, 209, 103298. [Google Scholar] [CrossRef]
- Liu, X.-M.; Teng, F.-Z.; Rudnick, R.L.; McDonough, W.F.; Cummings, M.L. Massive magnesium depletion and isotope fractionation in weathered basalts. Geochim. Cosmochim. Acta 2014, 135, 336–349. [Google Scholar] [CrossRef]
- Guo, B.; Zhu, X.; Dong, A.; Yan, B.; Shi, G.; Zhao, Z. Mg isotopic systematics and geochemical applications: A critical review. J. Asian Earth Sci. 2019, 176, 368–385. [Google Scholar] [CrossRef]
- Tipper, E.T.; Galy, A.; Bickle, M.J. Riverine evidence for a fractionated reservoir of Ca and Mg on the continents: Implications for the oceanic Ca cycle. Earth Planet. Sci. Lett. 2006, 247, 267–279. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, S.; Yang, C.; Guo, Y.; Xu, J.; Zhang, C. Mg isotopes of siliciclastic sediments on continental marginal sea: Insights for the potential to trace silicate weathering. Glob. Planet. Change 2023, 231, 104307. [Google Scholar] [CrossRef]
- Bristow, T.F.; Kennedy, M.J.; Morrison, K.D.; Mrofka, D.D. The influence of authigenic clay formation on the mineralogy and stable isotopic record of lacustrine carbonates. Geochim. Cosmochim. Acta 2012, 90, 64–82. [Google Scholar] [CrossRef]
- Deocampo, D.M. Chapter 1 The Geochemistry of Continental Carbonates. Dev. Sedimentol. 2010, 62, 1–59. [Google Scholar] [CrossRef]
- Jones, B.F.; Deocampo, D.M. Geochemistry of Saline Lakes. Treatise Geochem. 2013, 437, 393–424. [Google Scholar]
- Shalev, N.; Lazar, B.; Halicz, L.; Gavrieli, I. The Mg isotope signature of marine Mg-evaporites. Geochim. Cosmochim. Acta 2021, 301, 30–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, X.; Zhang, H. Sedimentary characteristics and formation mechanism of peneprimary dolostone in the Upper Eocene salt-bearing interval in Qianjiang Sag, Jianghan Basin. J. Palaeogeogr. 2006, 8, 441–455. [Google Scholar]
- Li, W.; Beard, B.L.; Li, C.; Xu, H.; Johnson, C.M. Experimental calibration of Mg isotope fractionation between dolomite and aqueous solution and its geological implications. Geochim. Cosmochim. Acta 2015, 157, 164–181. [Google Scholar] [CrossRef]







| Elute | Volume (mL) | Comments |
|---|---|---|
| Milli Q H2O | 15~20 | Backwash to remove air bubbles and reduce resin compaction |
| 4 N HNO3 + 0.5 N HF | 4 | Cleaning resin alternatively for three times |
| Milli-Q H2O | 4 | |
| 2 N HNO3 | 2 × 2 | Resin conditioning |
| Sample in 2 N HNO3 | 1 | Sample loading |
| 2 N HNO3 + 0.5 HF | 5 | Removing matrix |
| 1 N HNO3 | 8 | |
| 1 N HNO3 | 1 | Before cut collection |
| 1 N HNO3 | 24 | Mg Collection |
| 1 N HNO3 | 1 | Post cut collection |
| Milli Q H2O | 5 | Recovering resin |
| Sample No. | Depth | Carb | CIA | [Mg2+]/ [Ca2+] | Mncarb | Fecarb | Mn/Sr | δ13Ccarb | δ18Ocarb | δ26Mgdol | δ26Mgsi | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | % | % | (ppm) | (%) | |||||||||
| L1-17 | 3030.80 | 15.97 | 58.80 | 0.42 | 1318.00 | 3.15 | 1.28 | −5.17 | −6.53 | −1.21 | 0.003 | 0.72 | 0.720 |
| L1-16 | 3031.50 | 14.27 | 63.33 | 0.74 | 1042.12 | 2.24 | 3.72 | −3.66 | −3.98 | −0.72 | 0.044 | 1.50 | 1.504 |
| L1-15 | 3031.60 | 34.95 | 66.09 | 0.42 | 1172.00 | 2.40 | 1.57 | −3.53 | −3.87 | −0.94 | 0.017 | - | - |
| L1-14 | 3031.90 | 5.91 | 64.00 | 0.84 | 1091.97 | 1.59 | 0.76 | −4.21 | −3.87 | −1.13 | 0.006 | 1.14 | 1.138 |
| L1-13 | 3032.80 | 9.83 | 64.41 | 0.88 | 1237.28 | 1.82 | 3.22 | - | - | −0.92 | 0.018 | 1.46 | 1.460 |
| L1-12 | 3033.90 | 3.00 | 63.95 | 1.05 | 888.20 | 1.28 | 1.56 | −4.81 | −2.91 | −0.86 | 0.053 | −0.03 | −0.026 |
| L1-11 | 3034.80 | 6.46 | 66.84 | 0.97 | 1348.56 | 2.00 | 3.62 | - | - | −0.83 | 0.033 | 1.05 | 1.055 |
| L1-10 | 3035.40 | 2.77 | 61.65 | 0.80 | 957.10 | 2.03 | 1.45 | −4.06 | −3.28 | −1.31 | 0.011 | −0.33 | −0.326 |
| L1-9 | 3037.30 | 7.93 | 65.53 | 0.97 | 1335.40 | 2.10 | 4.04 | −3.81 | −3.47 | −1.50 | 0.053 | 0.47 | 0.466 |
| L1-8 | 3039.40 | 8.45 | 63.08 | 1.00 | 1260.20 | 2.18 | 3.03 | −3.58 | −3.32 | −1.47 | 0.045 | −0.10 | −0.104 |
| L1-7 | 3040.20 | 5.69 | 64.97 | 1.30 | 1122.79 | 1.85 | 2.27 | - | - | −1.76 | 0.057 | −0.71 | −0.712 |
| L1-6 | 3042.00 | 46.51 | 64.30 | 1.02 | 815.19 | 1.93 | 3.14 | −5.32 | −2.21 | - | - | - | - |
| L1-5 | 3042.30 | 17.77 | 63.78 | 0.98 | 727.74 | 1.32 | 2.94 | −5.21 | −4.15 | −1.34 | 0.019 | 0.56 | 0.559 |
| L1-4 | 3044.70 | 15.52 | 69.01 | 0.71 | 1013.19 | 0.53 | 1.00 | −3.57 | −5.24 | −2.32 | 0.055 | 0.37 | 0.373 |
| L1-3 | 3045.20 | 7.51 | 70.60 | 0.86 | 1405.07 | 0.47 | 1.09 | −3.66 | −3.98 | −2.40 | 0.052 | 0.34 | 0.337 |
| L1-2 | 3046.10 | 15.45 | 68.82 | 0.09 | 747.24 | 0.35 | 1.73 | - | - | −2.34 | 0.020 | 0.50 | 0.505 |
| L1-1 | 3046.70 | 13.94 | 72.10 | 0.91 | 1390.01 | 2.07 | 4.64 | −3.69 | −3.92 | −1.82 | 0.042 | 0.31 | 0.306 |
| GSR-6 | −1.76 | 0.008 | −0.915 | 0.030 | |||||||||
| GSR-12 | −2.08 | 0.002 | −1.042 | 0.047 | |||||||||
| GSR-30 | −1.90 | 0.018 | −2.022 | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Ling, K.; Wei, R.; Dong, L. Dynamic Climate Influence on Magnesium Isotope Variation in Saline Lacustrine Dolomite: A Case Study of the Qianjiang Formation, Jianghan Basin. Minerals 2024, 14, 459. https://0-doi-org.brum.beds.ac.uk/10.3390/min14050459
Wang T, Ling K, Wei R, Dong L. Dynamic Climate Influence on Magnesium Isotope Variation in Saline Lacustrine Dolomite: A Case Study of the Qianjiang Formation, Jianghan Basin. Minerals. 2024; 14(5):459. https://0-doi-org.brum.beds.ac.uk/10.3390/min14050459
Chicago/Turabian StyleWang, Tianyu, Kun Ling, Ren Wei, and Lin Dong. 2024. "Dynamic Climate Influence on Magnesium Isotope Variation in Saline Lacustrine Dolomite: A Case Study of the Qianjiang Formation, Jianghan Basin" Minerals 14, no. 5: 459. https://0-doi-org.brum.beds.ac.uk/10.3390/min14050459