Onion Polyphenols as Multi-Target-Directed Ligands in MASLD: A Preliminary Molecular Docking Study
Abstract
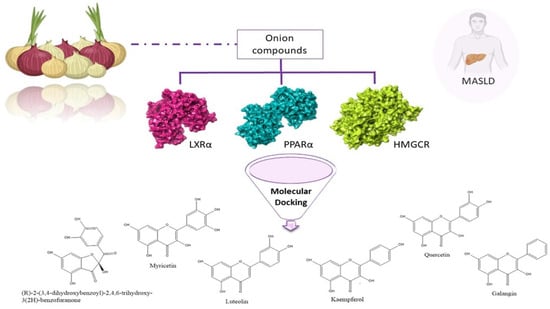
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaffner, F.; Thaler, H. Nonalcoholic fatty liver disease. Prog. Liver Dis. 1986, 8, 283–298. [Google Scholar] [PubMed]
- Abenavoli, L.; Giubilei, L.; Procopio, A.C.; Spagnuolo, R.; Luzza, F.; Boccuto, L.; Scarpellini, E. Gut Microbiota in Non-Alcoholic Fatty Liver Disease Patients with Inflammatory Bowel Diseases: A Complex Interplay. Nutrients 2022, 14, 5323. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, A.; Procopio, A.C.; Primerano, F.; Larussa, T.; Luzza, F.; Di Renzo, L.; De Lorenzo, A.; Gualtieri, P.; Abenavoli, L. Beneficial effects of coffee in non-alcoholic fatty liver disease: A narrative review. Hepatoma Res. 2020, 6, 69. [Google Scholar] [CrossRef]
- Larussa, T.; Abenavoli, L.; Procopio, A.C.; Iannelli, C.; Polimeni, N.; Spagnuolo, R.; Doldo, P.; Luzza, F. The role of gluten-free diet in nonalcoholic fatty liver disease development. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6613–6618. [Google Scholar] [CrossRef]
- Abenavoli, L.; Procopio, A.C.; Paravati, M.R.; Costa, G.; Milić, N.; Alcaro, S.; Luzza, F. Mediterranean Diet: The Beneficial Effects of Lycopene in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2022, 11, 3477. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Maurizi, V.; Boccuto, L.; Di Berardino, A.; Giostra, N.; Santori, P.; Scarcella, M.L.; Procopio, A.C.; Rasetti, C.; Scarpellini, E. Nutritional Support in Acute Liver Failure. Diseases 2022, 10, 108. [Google Scholar] [CrossRef]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological Properties of Allium cepa, Preclinical and Clinical Evidences; A Review. Iran. J. Pharm. Res. 2021, 20, 107–134. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Chen, X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn. Mag. 2010, 6, 135–141. [Google Scholar] [CrossRef]
- Xu, T.; Huang, S.; Huang, Q.; Ming, Z.; Wang, M.; Li, R.; Zhao, Y. Kaempferol attenuates liver fibrosis by inhibiting activin receptor–like kinase 5. J. Cell. Mol. Med. 2019, 23, 6403–6410. [Google Scholar] [CrossRef]
- Xia, S.F.; Le, G.W.; Wang, P.; Qiu, Y.Y.; Jiang, Y.Y.; Tang, X. Regressive effect of myricetin on hepatic steatosis in mice fed a high-fat diet. Nutrients 2016, 8, 799. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Ohmuma, M. Effects of isorhamnetin, rhamnetin, and quercetin on the concentrations of cholesterol and lipoperoxide in the serum and liver and on the blood and liver antioxidative enzyme activities of rats. Biosci. Biotechnol. Biochem. 1995, 59, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Chen, X.; Wu, J.; Lin, B.; Zhang, H.; Lan, L.; Luo, H. Galangin inhibits proliferation of hepatocellular carcinoma cells by inducing endoplasmic reticulum stress. Food Chem. Toxicol. 2013, 62, 810–816. [Google Scholar] [CrossRef]
- Simón, J.; Casado-Andrés, M.; Goikoetxea-Usandizaga, N.; Serrano-Maciá, M.; Martínez-Chantar, M.L. Nutraceutical Properties of Polyphenols against Liver Diseases. Nutrients 2020, 12, 3517. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, P.; Wang, T.; Wang, X.; Zheng, W.; Li, J. Baicalein reduces hepatic fat accumulation by activating AMPK in oleic acid-induced HepG2 cells and high-fat diet-induced non-insulin-resistant mice. Food Funct. 2020, 11, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Gao, L.; Lin, H.; Wu, Y.; Han, X.; Zhu, Y.; Li, J. Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochem. Biophys. Res. Commun. 2017, 482, 720–726. [Google Scholar] [CrossRef]
- Kim, J.D.; Liu, L.; Guo, W.; Meydani, M. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J. Nutr. Biochem. 2006, 17, 165–176. [Google Scholar] [CrossRef]
- Istvan, E.S. Structural mechanism for statin inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Am. Heart J. 2002, 144, S27–S32. [Google Scholar] [CrossRef]
- Fatima, K.; Moeed, A.; Waqar, E.; Atif, A.R.; Kamran, A.; Rizvi, H.; Suri, N.F.; Haider, H.; Shuja, S.H.; Khalid, M.; et al. Efficacy of statins in treatment and development of non-alcoholic fatty liver disease and steatohepatitis: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101816. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, S.G. Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction. Diabetes Metab. J. 2020, 44, 213–221. [Google Scholar] [CrossRef]
- Zhou, S.; You, H.; Qiu, S.; Yu, D.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. A new perspective on NAFLD: Focusing on the crosstalk between peroxisome proliferator-activated receptor alpha (PPARα) and farnesoid X receptor (FXR). Biomed. Pharmacother. 2022, 154, 113577. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, C.; Kim, T.H. Targeting Liver X Receptors for the Treatment of Non-Alcoholic Fatty Liver Disease. Cells 2023, 12, 1292. [Google Scholar] [CrossRef]
- The Metabolomics Innovation Centre. FooDB, Version 1. Available online: http://foodb.ca/ (accessed on 6 November 2023).
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef] [PubMed]
- ChemDraw Professional. ChemDraw Professional, Version 16 0.0.82 (68); Perkin Elmer Informatics, Inc.: Cambridge, MA, USA, 2019.
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Schrödinger. Free Maestro Academic, Version v13.4 Package; Schrödinger Release 2022-4: Maestro; Schrödinger, LLC: New York, NY, USA, 2022. [Google Scholar]
- da Costa, R.F.; Freire, V.N.; Bezerra, E.M.; Cavada, B.S.; Caetano, E.W.; de Lima Filho, J.L.; Albuquerque, E.L. Explaining statin inhibition effectiveness of HMG-CoA reductase by quantum biochemistry computations. Phys. Chem. Chem. Phys. 2012, 14, 1389–1398. [Google Scholar] [CrossRef]
- Svensson, S.; Ostberg, T.; Jacobsson, M.; Norström, C.; Stefansson, K.; Hallén, D.; Johansson, I.C.; Zachrisson, K.; Ogg, D.; Jendeberg, L. Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand-binding domains in a fully agonistic conformation. EMBO J. 2003, 22, 4625–4633. [Google Scholar] [CrossRef] [PubMed]
- Kamata, S.; Honda, A.; Ishikawa, R.; Akahane, M.; Fujita, A.; Kaneko, C.; Miyawaki, S.; Habu, Y.; Shiiyama, Y.; Uchii, K.; et al. Functional and Structural Insights into the Human PPARα/δ/γ Targeting Preferences of Anti-NASH Investigational Drugs, Lanifibranor, Seladelpar, and Elafibranor. Antioxidants 2023, 12, 1523. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Machado, M.V. MASLD treatment-a shift in the paradigm is imminent. Front. Med. 2023, 10, 1316284. [Google Scholar] [CrossRef]
- Feng, C.; Han, A.; Ye, C.; Xu, R.; Li, M. The HMG-CoA reductase pathway, statins and angioprevention. Semin. Ophthalmol. 2006, 21, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H. Functional Implications of HMG-CoA Reductase Inhibition on Glucose Metabolism. Korean Circ. J. 2018, 48, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, M.; Rodrigues-Díez, R.; Blanco-Colio, L.M.; Sánchez-López, E.; Rodríguez-Vita, J.; Esteban, V.; Carvajal, G.; Plaza, J.J.; Egido, J.; Ruiz-Ortega, M. HMG-CoA reductase inhibitors decrease angiotensin II-induced vascular fibrosis: Role of RhoA/ROCK and MAPK pathways. Hypertension 2007, 50, 377–383. [Google Scholar] [CrossRef]
- Galiero, R.; Caturano, A.; Vetrano, E.; Cesaro, A.; Rinaldi, L.; Salvatore, T.; Marfella, R.; Sardu, C.; Moscarella, E.; Gragnano, F.; et al. Pathophysiological mechanisms and clinical evidence of relationship between Nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease. Rev. Cardiovasc. Med. 2021, 22, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Poulsen, K.L.; Wu, L.; Liu, S.; Miyata, T.; Song, Q.; Wei, Q.; Zhao, C.; Lin, C.; Yang, J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target. Ther. 2022, 7, 287. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. The structure of the catalytic portion of human HMG-CoA reductase. Biochim. Biophys. Acta 2000, 1529, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef]
- da Silva, V.B.; Taft, C.A.; Silva, C.H. Use of virtual screening, flexible docking, and molecular interaction fields to design novel HMG-CoA reductase inhibitors for the treatment of hypercholesterolemia. J. Phys. Chem. A 2008, 112, 2007–2011. [Google Scholar] [CrossRef]
- Waiz, M.; Alvi, S.S.; Khan, M.S. Potential dual inhibitors of PCSK-9 and HMG-R from natural sources in cardiovascular risk management. EXCLI J. 2022, 21, 47–76. [Google Scholar] [CrossRef]
- Khamis, A.A.; Salama, A.F.; Kenawy, M.E.; Mohamed, T.M. Regulation of hepatic hydroxy methyl glutarate—CoA reductase for controlling hypercholesterolemia in rats. Biomed. Pharmacother. 2017, 95, 1242–1250. [Google Scholar] [CrossRef]
- Cariello, M.; Piccinin, E.; Moschetta, A. Transcriptional Regulation of Metabolic Pathways via Lipid-Sensing Nuclear Receptors PPARs, FXR, and LXR in NASH. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1519–1539. [Google Scholar] [CrossRef] [PubMed]
- Dixon, E.D.; Nardo, A.D.; Claudel, T.; Trauner, M. The Role of Lipid Sensing Nuclear Receptors (PPARs and LXR) and Metabolic Lipases in Obesity, Diabetes and NAFLD. Genes 2021, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Tice, C.M.; Noto, P.B.; Fan, K.Y.; Zhuang, L.; Lala, D.S.; Singh, S.B. The medicinal chemistry of liver X receptor (LXR) modulators. J. Med. Chem. 2014, 57, 7182–7205. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, K.; Kim, S.H.; Hong, M.J.; Jeong, N.J.; Kim, M.S. Luteolin improves hypercholesterolemia and glucose intolerance through LXRα-dependent pathway in diet-induced obese mice. J. Food Biochem. 2020, 44, e13358. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Schoonjans, K.; Lefebvre, A.M.; Staels, B.; Auwerx, J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J. Biol. Chem. 1997, 272, 28210–28217. [Google Scholar] [CrossRef] [PubMed]
- Gulick, T.; Cresci, S.; Caira, T.; Moore, D.D.; Kelly, D.P. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc. Natl. Acad. Sci. USA 1994, 91, 11012–11016. [Google Scholar] [CrossRef] [PubMed]
- Hebbachi, A.M.; Knight, B.L.; Wiggins, D.; Patel, D.D.; Gibbons, G.F. Peroxisome proliferator-activated receptor alpha deficiency abolishes the response of lipogenic gene expression to re-feeding: Restoration of the normal response by activation of liver X receptor alpha. J. Biol. Chem. 2008, 283, 4866–4876. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, R.M.; Baugé, E.; Staels, B.; Gervois, P. Systemic and distal repercussions of liver-specific peroxisome proliferator-activated receptor-alpha control of the acute-phase response. Endocrinology 2008, 149, 3215–3223. [Google Scholar] [CrossRef]
- Guerre-Millo, M.; Gervois, P.; Raspé, E.; Madsen, L.; Poulain, P.; Derudas, B.; Herbert, J.M.; Winegar, D.A.; Willson, T.M.; Fruchart, J.C.; et al. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 2000, 275, 16638–16642. [Google Scholar] [CrossRef]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ip, E.; Farrell, G.; Hall, P.; Robertson, G.; Leclercq, I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 2004, 39, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Dhoke, G.V.; Gangwal, R.P.; Sangamwar, A.T. A combined ligand and structure based approach to design potent PPAR-alpha agonists. J. Mol. Struct. 2012, 1028, 22–30. [Google Scholar] [CrossRef]




| Compound | LXR-α | LXR-β | PPAR-α | PPAR-γ | HMGCR |
|---|---|---|---|---|---|
| Quercetin | 53.096 | 52.201 | 67.934 | 64.722 | 57.461 |
| Kaempferol | 51.474 | 52.585 | 58.782 | 65.393 | 51.729 |
| Galangin | 54.240 | 55.374 | 60.412 | 65.263 | 50.591 |
| Baicalein | 54.319 | 51.875 | 70.611 | 68.185 | 51.099 |
| Luteolin | 53.643 | 52.417 | 66.962 | 65.680 | 54.989 |
| Myricetin | 55.083 | 53.265 | 65.655 | 59.200 | 58.348 |
| Isorhamnetin | 54.087 | 50.041 | 54.434 | 62.746 | 50.958 |
| Benzofuranone | 52.215 | 52.927 | 58.102 | 69.113 | 58.726 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paravati, M.R.; Procopio, A.C.; Milanović, M.; Scarlata, G.G.M.; Milošević, N.; Ružić, M.; Milić, N.; Abenavoli, L. Onion Polyphenols as Multi-Target-Directed Ligands in MASLD: A Preliminary Molecular Docking Study. Nutrients 2024, 16, 1226. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16081226
Paravati MR, Procopio AC, Milanović M, Scarlata GGM, Milošević N, Ružić M, Milić N, Abenavoli L. Onion Polyphenols as Multi-Target-Directed Ligands in MASLD: A Preliminary Molecular Docking Study. Nutrients. 2024; 16(8):1226. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16081226
Chicago/Turabian StyleParavati, Maria Rosaria, Anna Caterina Procopio, Maja Milanović, Giuseppe Guido Maria Scarlata, Nataša Milošević, Maja Ružić, Nataša Milić, and Ludovico Abenavoli. 2024. "Onion Polyphenols as Multi-Target-Directed Ligands in MASLD: A Preliminary Molecular Docking Study" Nutrients 16, no. 8: 1226. https://0-doi-org.brum.beds.ac.uk/10.3390/nu16081226