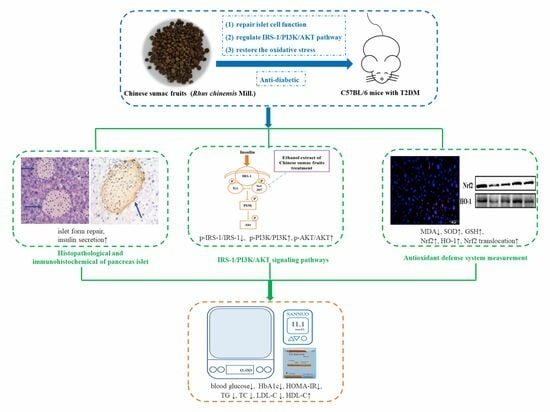
Chinese Sumac Fruits (Rhus chinesis Mill.) Alleviate Type 2 Diabetes in C57BL/6 Mice through Repairing Islet Cell Functions, Regulating IRS-1/PI3K/AKT Pathways and Promoting the Entry of Nrf2 into the Nucleus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Samples and Identification of Phytochemica
2.3. Experimental Design of Animals
2.4. Glucose Tolerance Test Administered Orally (OGTT) and Insulin Tolerance Test Administered Intraperitoneally (ITT)
2.5. Biochemical Analysis of Plasma and Liver
2.6. Histopathological and Immunohistochemical Analyses
2.7. Immunofluorescence Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Extracts of R. chinensis Mill. Reduce Energy Intake in T2D Mice
3.2. Extracts of R. chinensis Mill. Effectively Lower the Blood Glucose Levels in T2D Mice
3.3. Extracts of R. chinensis Mill. Regulate OGTT and ITT in T2DM Mice
3.4. Extracts of R. chinensis Mill. Exhibit Beneficial Effects on Lipid Metabolism
3.5. Extracts of R. chinensis Mill. Repair Islet Cell Structure and Function
3.6. Extracts of R. chinensis Mill. Protect the Antioxidant Defense System in T2D Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meetoo, D.; McGovern, P.; Safadi, R. An epidemiological overview of diabetes across the world. Br. J. Nurs. 2013, 16, 1002–1007. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besanon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, J.; Ding, F.; Wu, X.; Pan, C.; Wang, Q.; Gao, M.; Duan, S.; Han, X.; Xia, K.; et al. Maca extracts regulate glucose and lipid metabolism in insulin-resistant HepG2 cells via the PI3K/AKT signalling pathway. Food Sci. Nutr. 2021, 9, 2894–2907. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jayachandran, M.; Xu, B. Antidiabetic effect of konjac glucomannan via insulin signaling pathway regulation in high-fat diet and streptozotocin-induced diabetic rats. Food Res. Int. 2021, 149, 110664. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, M.; Zhou, H.; Cheng, L.; Wei, X.; Wang, Y. Liubao brick tea activates the PI3K-AKT signaling pathway to lower blood glucose, metabolic disorders and insulin resistance via altering the intestinal flora. Food Res. Int. 2021, 148, 110594. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-Q.; Chen, H.-H.; Zhang, J.-Y.; Zhang, Y.-J.; Yang, J.-W.; Pan, H.-J.; Song, W.-X.; Murad, F.; He, Y.-Q.; Bian, K. Rutaecarpine ameliorates hyperlipidemia and hyperglycemia in fat-fed, streptozotocin-treated rats via regulating the IRS-1/PI3K/Akt and AMPK/ACC2 signaling pathways. Acta Pharmacol. Sin. 2016, 37, 483–496. [Google Scholar] [CrossRef]
- Matsuzawa-Nagata, N.; Takamura, T.; Ando, H.; Nakamura, S.; Kurita, S.; Misu, H.; Ota, T.; Yokoyama, M.; Honda, M.; Miyamoto, K.-I.; et al. Increased oxidative stress precedes the onset of high-fat diet–induced insulin resistance and obesity. Metabolism 2008, 57, 1071–1077. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef]
- Dyson, P.; Kelly, T.; Deakin, T.; Duncan, A.; Frost, G.; Harrison, Z.; Khatri, D.; Kunka, D.; McArdle, P.; Mellor, D. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2011, 28, 1282–1288. [Google Scholar] [CrossRef]
- Toi, P.L.; Anothaisintawee, T.; Chaikledkaew, U.; Briones, J.R.; Reutrakul, S.; Thakkinstian, A. Preventive role of diet interventions and dietary factors in type 2 diabetes mellitus: An umbrella review. Nutrients 2020, 12, 2722. [Google Scholar] [CrossRef] [PubMed]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-week mediterranean diet intervention increases citrus bioflavonoid levels and reduces inflammation in people with type 2 diabetes mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Ganugapati, J.; Baldwa, A.; Lalani, S. Molecular docking studies of banana flower flavonoids as insulin receptor tyrosine kinase activators as a cure for diabetes mellitus. Bioinformation 2012, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tan, S.; Ren, T.; Wang, H.; Dai, X.; Wang, H. Polyphenol from Rosa roxburghii Tratt fruit ameliorates the symptoms of diabetes by activating the P13K/AKT insulin pathway in db/db mice. Foods 2022, 11, 636. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla Chinensis–folklore to modern evidence: Review. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhao, Y.; Hong, Y.; Cai, S.; Pang, M. Phenolic composition, antioxidant and pancreatic lipase inhibitory activities of Chinese sumac (Rhus chinensis Mill.) fruits extracted by different solvents and interaction between myricetin-3-O-rhamnoside and quercetin-3-O-rhamnoside. Int. J. Food Sci. Technol. 2018, 53, 1045–1053. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.; Ma, Q.; Yi, J.; Cai, S. Anti-diabetic effects of different phenolic-rich fractions from Rhus chinensis Mill. fruits in vitro. eFood 2021, 2, 37–46. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, X.; Ma, Q.; Yi, J.; Cai, S. Phytochemical bioaccessibility and in vitro antidiabetic effects of Chinese sumac (Rhus chinensis Mill.) fruits after a simulated digestion: Insights into the mechanisms with molecular docking analysis. Int. J. Food Sci. Technol. 2022, 57, 2656–2669. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Y.; Zhao, L.; Cai, S.; Cheng, G. Acute and subchronic toxicities of the ethanol and hot-water extracts from Chinese sumac (Rhus chinensis Mill.) fruits by oral administration in rats. Food Chem. Toxicol. 2018, 119, 14–23. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Wang, J.; Yang, H.; Li, S.; Jiang, W.; Liu, Y.; Li, J. Long-chain bases from sea cucumber inhibits renal fibrosis and apoptosis in type 2 diabetic mice. J. Funct. Foods 2018, 40, 760–768. [Google Scholar] [CrossRef]
- Luo, W.; Zhou, J.; Yang, X.; Wu, R.; Liu, H.; Shao, H.; Huang, B.; Kang, X.; Yang, L.; Liu, D. A Chinese medical nutrition therapy diet accompanied by intermittent energy restriction alleviates type 2 diabetes by enhancing pancreatic islet function and regulating gut microbiota composition. Food Res. Int. 2022, 161, 111744. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ma, Y.; Gong, X.; Zhang, Y.; Zhao, L.; Cheng, G.; Cai, S. Rhus chinensis Mill. fruits prevent high-fat/ethanol diet-induced alcoholic fatty liver in rats via AMPK/SREBP-1/FAS signaling pathway. J. Funct. Foods 2019, 61, 103498. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, F.; Chen, Y.; Zhang, Y.; Hou, L.; Cao, X.; Wang, C. A polysaccharide from Grifola frondose relieves insulin resistance of HepG2 cell by Akt-GSK-3 pathway. Glycoconj. J. 2014, 31, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Mayerson, A.B.; Hundal, R.S.; Dufour, S.; Lebon, V.; Befroy, D.; Lebon, V.; Befroy, D.; Cline, G.W.; Enocksson, S.; Inzucchi, S.E.; et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 2002, 51, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Lachin, J.M.; Zinman, B.; Haffner, S.M.; Aftring, R.P.; Paul, G.; Kravitz, B.G.; Herman, W.H.; Vibrti, G.; Holman, R.R.; et al. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 2011, 60, 1552–1560. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Ferrannini, E.; Miyazaki, Y.; Matsuda, M.; Mari, A.; DeFronzo, R.A. Thiazolidinediones improve β-cell function in type 2 diabetic patients. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E871–E883. [Google Scholar] [CrossRef]
- Munteanu, C.; Mihai, M.; Dulf, F.; Ona, A.; Muntean, L.; Ranga, F.; Urdă, C.; Pop, D.; Mihaiescu, T.; Mârza, S.M.; et al. Biochemical changes induced by the administration of Cannabis sativa seeds in diabetic Wistar rats. Nutrients 2023, 15, 2944. [Google Scholar] [CrossRef]
- Carmona, M.C.; Louche, K.; Nibbelink, M.; Prunet, B.; Bross, A.; Desbazeille, M.; Dacquet, C.; Renard, P.; Casteilla, L.; Penicaud, L. Fenofibrate prevents Rosiglitazone-induced body weight gain in ob/ob mice. Int. J. Obes. 2005, 29, 864–871. [Google Scholar] [CrossRef]
- Chaput, E.; Saladin, R.; Silvestre, M.; Edgar, A.D. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem. Biophys. Res. Commun. 2000, 271, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.B.; Xu, X.M.; Shu, Z.Z.; Li, L.F.; Chen, Z.J. Blood glucose not hemoglobin influenced glycosylated hemoglobin in type 2 diabetes patients on plateau of China. Int. J. Diabetes Dev. C 2015, 35, 197–200. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Sun, Z.; Yan, X.; Wang, H.; Xu, H.; Ma, J.; Zhang, Y. Linderane protects pancreatic β cells from streptozotocin (STZ)-induced oxidative damage. Life Sci. 2019, 233, 116732. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Fang, J.; Guo, H.; Su, X.; Zhu, B.; Yao, X.; Wang, Y.; Cao, A.; Wang, H.; Wang, L. Astragaloside IV attenuates podocyte apoptosis through ameliorating mitochondrial dysfunction by up-regulated Nrf2-ARE/TFAM signaling in diabetic kidney disease. Free Radic. Biol. Med. 2023, 203, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, N.; Yi, J.; Zhou, L.; Cai, S. Gastroprotective effect and mechanisms of Chinese sumac fruits (Rhus chinensis Mill.) on ethanol-induced gastric ulcers in mice. Food Funct. 2021, 12, 12565–12579. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) improves antioxidant capacity via PI3K/AKT pathways and Nrf2 translocation in a type 2 diabetes model. Molecules 2019, 24, 1906. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Shi, S.; Gao, S.; Wang, Y.; Xiao, D.; Chen, T.; He, Q.; Zhang, J.; Lin, Y. Tetrahedral framework nucleic acids ameliorate insulin resistance in type 2 diabetes mellitus via the PI3K/Akt pathway. ACS Appl. Mater. Interfaces 2021, 13, 40354–40364. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483. [Google Scholar] [CrossRef]
- Camaya, I.; Donnelly, S.; O’Brien, B. Targeting the PI3K/Akt signaling pathway in pancreatic β-cells to enhance their survival and function: An emerging therapeutic strategy for type 1 diabetes. J. Diabetes 2022, 14, 247–260. [Google Scholar] [CrossRef]
- Tong, T.; Ren, N.; Soomi, P.; Wu, J.; Guo, N.; Kang, H.; Kim, E.; Wu, Y.; He, P.; Tu, Y.; et al. Theaflavins improve insulin sensitivity through regulating mitochondrial biosynthesis in palmitic acid-induced HepG2 cells. Molecules 2018, 23, 3382. [Google Scholar] [CrossRef]
- Jahandideh, F.; de Campos Zani, S.C.; Son, M.; Proctor, S.D.; Davidge, S.T.; Chan, C.B.; Wu, J. Egg white hydrolysate enhances insulin sensitivity in high fat diet induced insulin resistant rats via AKT activation. Br. J. Nutr. 2019, 122, 14–24. [Google Scholar] [CrossRef]
- Fernández-Millán, E.; Martín, M.A.; Goya, L.; Lizárraga-Mollinedo, E.; Escrivá, F.; Ramos, S.; Álvarez, C. Glucagon-like peptide-1 improves beta-cell antioxidant capacity via extracellular regulated kinases pathway and Nrf2 translocation. Free Radic. Biol. Med. 2016, 95, 16–26. [Google Scholar] [CrossRef]
- Lozano, I.; Werf, R.V.D.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Kukidome, D.; Sonoda, K.; Fujisawa, K.; Matsuhisa, T.; Motoshima, H.; Matsumura, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res. Clin. Pract. 2007, 77, S161–S164. [Google Scholar] [CrossRef] [PubMed]
- Boura-Halfon, S.; Zick, Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E581–E591. [Google Scholar] [CrossRef] [PubMed]









| Parameters | C | M | L | H | P |
|---|---|---|---|---|---|
| Weight in week 13 (g) | 23.09 ± 3.04 a | 29.37 ± 5.21 b | 29.05 ± 5.07 b | 29.21 ± 4.36 b | 29.72 ± 3.87 b |
| Weight in week 14 (g) | 23.29 ± 3.18 a | 26.41 ± 3.44 b | 27.06 ± 5.83 b | 26.47 ± 2.77 b | 26.23 ± 4.38 b |
| Weight in week 20 (g) | 25.60 ± 4.33 b | 23.87 ± 3.68 a | 22.49 ± 5.19 a | 21.84 ± 3.28 a | 27.38 ± 3.01 b |
| Energy intake (week 1–13) (kcal/week·g body weight) | 2.39 ± 0.33 a | 2.84 ± 1.12 b | 2.82 ± 0.69 b | 2.81 ± 0.69 b | 2.84 ± 0.96 b |
| Energy intake (week 14–20) (kcal/week·g body weight) | 2.66 ± 0.44 b | 2.48 ± 0.69 b | 2.02 ± 0.87 a | 2.04 ± 1.14 a | 2.62 ± 0.65 b |
| TG (mmol L−1) | 1.10 ± 0.11 a | 1.71 ± 0.27 c | 1.31 ± 0.17 a | 1.22 ± 0.48 a | 1.51 ± 0.21 b |
| TC (mmol L−1) | 1.75 ± 0.61 a | 4.38 ± 0.80 c | 3.78 ± 0.65 b | 3.29 ± 0.69 b | 3.51 ± 0.86 b |
| LDL-C (mmol L−1) | 0.39 ± 0.15 a | 1.21 ± 0.48 c | 0.71 ± 0.25 b | 0.50 ± 0.15 a | 0.55 ± 0.17 ab |
| HDL-C (mmol L−1) | 1.39 ± 0.17 c | 0.89 ± 0.26 a | 0.97 ± 0.33 a | 1.18 ± 0.18 b | 1.04 ± 0.26 b |
| Insulin (mU L−1) | 24.13 ± 4.07 c | 13.72 ± 3.20 a | 17.61 ± 3.17 b | 19.23 ± 4.07 b | 17.61 ± 3.36 b |
| HOMA-IR | 5.45 ± 0.95 a | 9.32 ± 1.78 c | 6.97 ± 1.95 b | 5.95 ± 0.69 ab | 6.11 ± 1.12 ab |
| HbA1c (ng mL−1) | 207.79 ± 18.49 a | 301.61 ± 53.60 c | 265.76 ± 29.26 bc | 236.36 ± 47.86 ab | 219.00 ± 32.14 a |
| GSH (nmol mg−1 prot) | 222.60 ± 32.82 d | 122.69 ± 29.02 a | 151.92 ± 18.80 ab | 192.09 ± 25.80 cd | 162.04 ± 36.49 bc |
| MDA (nmol mg−1 prot) | 7.92 ± 1.83 a | 14.16 ± 4.52 c | 10.63 ± 1.30 bc | 10.25 ± 0.89 ab | 12.10 ± 2.01 bc |
| SOD (U/mg prot) | 321.45 ± 57.15 c | 186.16 ± 30.00 a | 235.95 ± 37.26 ab | 279.80 ± 51.86 bc | 253.58 ± 55.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cai, S.; Yi, J.; Chu, C. Chinese Sumac Fruits (Rhus chinesis Mill.) Alleviate Type 2 Diabetes in C57BL/6 Mice through Repairing Islet Cell Functions, Regulating IRS-1/PI3K/AKT Pathways and Promoting the Entry of Nrf2 into the Nucleus. Nutrients 2023, 15, 4080. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15184080
Liu X, Cai S, Yi J, Chu C. Chinese Sumac Fruits (Rhus chinesis Mill.) Alleviate Type 2 Diabetes in C57BL/6 Mice through Repairing Islet Cell Functions, Regulating IRS-1/PI3K/AKT Pathways and Promoting the Entry of Nrf2 into the Nucleus. Nutrients. 2023; 15(18):4080. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15184080
Chicago/Turabian StyleLiu, Xiaojing, Shengbao Cai, Junjie Yi, and Chuanqi Chu. 2023. "Chinese Sumac Fruits (Rhus chinesis Mill.) Alleviate Type 2 Diabetes in C57BL/6 Mice through Repairing Islet Cell Functions, Regulating IRS-1/PI3K/AKT Pathways and Promoting the Entry of Nrf2 into the Nucleus" Nutrients 15, no. 18: 4080. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15184080