Regulation of Insulin Resistance, Lipid Profile and Glucose Metabolism Associated with Polycystic Ovary Syndrome by Tinospora cordifolia
Abstract
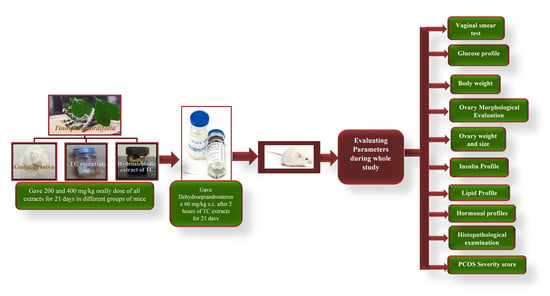
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Authentication
2.2. Reagents and Chemicals
2.3. Instruments
2.4. Preparation of Hydroalcoholic Extract
2.5. Formulation of Guduchi Satva
2.6. Isolation of Essential Oil
2.7. Animal Handling and Groups
2.8. Establishment of PCOS Model
2.9. Estrous Cycle Monitoring and Vaginal Smear Test
2.10. Change in Body Weight
2.11. Measurement of Fasting Blood Glucose (FBG) and Oral Glucose Tolerance Test (OGTT)
2.12. Blood Collection, Collection of Plasma, and Detection of Biochemical Indexes
2.13. Measurement of Weight, Size, and Morphological Changes in the Ovaries
2.14. Histological Examination
2.15. Severity of PCOS
2.16. Statistical Analysis
3. Results
3.1. Estrous Cycle Determination
3.2. Body Weight
3.3. Fasting Blood Glucose Level
3.4. Oral Glucose Tolerance Test
3.5. Hormonal Profile
3.6. LH and FSH Level
3.7. Lipid Profiles
3.8. Insulin Profile
3.9. Ovarian Size and Weight
3.10. Morphological Observation of Ovaries
3.11. Histopathological Examination of the Ovaries
3.12. Severity Score Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Abraham Gnanadass, S.; Divakar Prabhu, Y.; Valsala Gopalakrishnan, A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): An update. Arch. Gynecol. Obstet. 2021, 303, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kong, X.; Yin, H.; Zhang, W.; Wang, W. Effect of Hawthorn Leaf Flavonoids in Dehydroepiandrosterone-Induced Polycystic Ovary Syndrome in Rats. Pathobiology 2019, 86, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, A.; Mioni, R.; Sabbadin, C.; Dassie, F.; Parolin, M.; Vettor, R.; Barbot, M.; Scaroni, C. Pharmacological Approaches to Controlling Cardiometabolic Risk in Women with PCOS. Int. J. Mol. Sci. 2020, 21, 9554. [Google Scholar] [CrossRef]
- Abudawood, M.; Tabassum, H.; Alanazi, A.H.; Almusallam, F.; Aljaser, F.; Ali, M.N.; Alenzi, N.D.; Alanazi, S.T.; Alghamdi, M.A.; Altoum, G.H.; et al. Antioxidant status in relation to heavy metals induced oxidative stress in patients with polycystic ovarian syndrome (PCOS). Sci. Rep. 2021, 11, 22935. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, M.; Kim, E.J.; Lee, M.J.; Park, K.S.; Kim, S.H.; In, J.G.; Kwak, Y.S.; Park, D.H.; Cho, S.S.; et al. Korean Red Ginseng alleviates dehydroepiandrosterone-induced polycystic ovarian syndrome in rats via its antiinflammatory and antioxidant activities. J. Ginseng Res. 2020, 44, 790–798. [Google Scholar] [CrossRef]
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef]
- Sunand, K.; Yellow, M.; Naveen, P.; Deepika, Y.; Mohan, G.K.; Bakshi, V. Betel Leaf Extract Amends Dehydroepiandrosterone Induced PCOS Related Hormonal Abnormality and Histopathological Alterations in Rat Model. Pharmacogn. J. 2019, 11, 1442–1448. [Google Scholar] [CrossRef]
- Agarwal, S.; Ramamurthy, P.H.; Fernandes, B.; Rath, A.; Sidhu, P. Assessment of antimicrobial activity of different concentrations of Tinospora cordifolia against Streptococcus mutans: An in vitro study. Dent. Res. J. 2019, 16, 24–28. [Google Scholar]
- Duhan, P.; Bansal, P.; Rani, S. Isolation, identification and characterization of endophytic bacteria from medicinal plant Tinospora cordifolia. S. Afr. J. Bot. 2020, 134, 43–49. [Google Scholar] [CrossRef]
- Pachiappan, S.; Matheswaran, S.; Saravanan, P.P.; Muthusamy, G. Medicinal Plants for Polycystic Ovary Syndrome: A Review of Phytomedicine Research. Int. J. Herb. Med. 2017, 5, 78–80. Available online: https://www.florajournal.com/archives/2017/vol5issue2/PartB/5-6-30-753.pdf (accessed on 13 March 2023).
- Sharma, P.; Dwivedee, B.P.; Bisht, D.; Dash, A.K.; Kumar, D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 2019, 5, e02437. [Google Scholar] [CrossRef] [PubMed]
- Moka, M.K.; Sumithra, M. Computational Investigation of Isoquinoline Alkaloid from the Stems of Tinospora cordifolia against Polycystic Ovarian Syndrome. Res. Sq. 2022, 1–15. [Google Scholar] [CrossRef]
- Yu, J.; Ding, C.; Hua, Z.; Jiang, X.; Wang, C. Protective effects of berberine in a rat model of polycystic ovary syndrome mediated via the PI3K/AKT pathway. J. Obstet. Gynaecol. Res. 2021, 47, 1789–1803. [Google Scholar] [CrossRef]
- Devi, M.S.; Muralidharan, P.; Hari, R.; Lavanya, M.; Abiraamavalli, T. PCOS Modulatory Activity of Tinospora cordifolia leaves—An Insilico Approach. Biomed. Pharmacol. J. 2021, 14, 1125–1131. [Google Scholar] [CrossRef]
- Food Safety and Standards Authority of India, F. No. Std/SP-05/T (Nutraceutical-2022) [E-5184]. 2022. Available online: https://www.fssai.gov.in/upload/advisories/2022/03/6243ef28079ceDirection_Nutra_30_03_2022.pdf (accessed on 20 April 2023).
- Selvaraj, K.; Sivakumar, G.; Pillai, A.A.; Veeraraghavan, V.P.; Bolla, S.R.; Veeraraghavan, G.R.; Rengasamy, G.; Joseph, J.P.; Janardhana, P.B. Phytochemical screening, HPTLC fingerprinting and Invitro antioxidant activity of root extract of Asparagus racemosus. Pharmacogn. J. 2019, 11, 818–823. [Google Scholar] [CrossRef]
- Katara, A.; Garg, N.K.; Mathur, M. Separation and Identification of Anti-diabetic compounds in Tinospora cordifolia extract and Ayurvedic formulation Guduchi Satva by GCMS and FTIR study with Subsequent Evaluation of in-vitro Hypoglycemic Potential. Int. J. Pharm. Sci. Drug Res. 2021, 13, 183–189. [Google Scholar] [CrossRef]
- Naik, D.; Dandge, C.; Rupanar, S. Determination of chemical composition and evaluation of antioxidant activity of essential oil from Tinospora cordifolia (Willd.) Leaf. J. Essent. Oil Bear. Plants 2014, 17, 228–236. [Google Scholar] [CrossRef]
- OECD Guidance Document on the Recognition, Assessment and Use of Clinical Signs as Human Endpoints for Experimental Animals Used in Safety Evaluation; OECD Series on Testing and Assessment, No. 19; OECD Publishing: Paris, France, 2002. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gd19.pdf (accessed on 12 February 2023).
- Rani, R.; Chitme, H.; Sharma, A.K. Effect of Tinospora cordifolia on gestational diabetes mellitus and its complications. Women Health 2023, 1–11. [Google Scholar] [CrossRef]
- Younas, A.; Hussain, L.; Shabbir, A.; Asif, M.; Hussain, M.; Manzoor, F. Effects of Fagonia indica on Letrozole-Induced Polycystic Ovarian Syndrome (PCOS) in Young Adult Female Rats. Evid. Based Complement. Alternat. Med. 2022, 2022, 1397060. [Google Scholar] [CrossRef]
- Dou, L.; Zheng, Y.; Li, L.; Gui, X.; Chen, Y.; Yu, M.; Guo, Y. The effect of cinnamon on polycystic ovary syndrome in a mouse model. Reprod. Biol. Endocrinol. 2018, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Soto, S.; Ornelas-Mendoza, K.; Navarrete-Vázquez, G.; Chávez-Silva, F.; Almanza-Pérez, J.C.; Villalobos-Molina, R.; Ortiz-Barragán, E.; Loza-Rodríguez, H.; Rivera-Leyva, J.C.; Flores-Flores, A.; et al. Insulin Sensitization by PPARγ and GLUT-4 Overexpression/Translocation Mediates the Antidiabetic Effect of Plantago australis. Pharmaceuticals 2023, 16, 535. [Google Scholar] [CrossRef] [PubMed]
- Kakadia, N.; Patel, P.; Deshpande, S.; Shah, G. Effect of Vitex negundo L. seeds in letrozole induced polycystic ovarian syndrome. J. Tradit. Complement. Med. 2018, 9, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Khachab, M.; Zaatiti, H.; Kanaan, A. Magnolia officinalis ameliorates dehydroepiandrosterone-induced polycystic ovary syndrome in rats. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e106447. [Google Scholar] [CrossRef]
- Mansour, A.; Hashemi Taheri, A.P.; Moradi, B.; Mohajeri-Tehrani, M.R.; Qorbani, M.; Ghorbani Pashakolaee, S.; Sanginabadi, M.; Sajjadi-Jazi, S.M. Ovarian volume, not follicle count, is independently associated with androgens in patients with polycystic ovary syndrome. BMC Endocr. Disord. 2022, 22, 298. [Google Scholar] [CrossRef]
- Rababa’h, A.M.; Matani, B.R.; Ababneh, M.A. The ameliorative effects of marjoram in dehydroepiandrosterone induced polycystic ovary syndrome in rats. Life Sci. 2020, 261, 118353. [Google Scholar] [CrossRef]
- Jozkowiak, M.; Piotrowska-Kempisty, H.; Kobylarek, D.; Gorska, N.; Mozdziak, P.; Kempisty, B.; Rachon, D.; Spaczynski, R.Z. Endocrine Disrupting Chemicals in Polycystic Ovary Syndrome: The Relevant Role of the Theca and Granulosa Cells in the Pathogenesis of the Ovarian Dysfunction. Cells 2022, 12, 174. [Google Scholar] [CrossRef]
- Ng, N.Y.H.; Jiang, G.; Cheung, L.P.; Zhang, Y.; Tam, C.H.T.; Luk, A.O.Y.; Quan, J.; Lau, E.S.H.; Yau, T.T.L.; Chan, M.H.M.; et al. Progression of glucose intolerance and cardiometabolic risk factors over a decade in Chinese women with polycystic ovary syndrome: A case-control study. PLoS Med. 2019, 16, e1002953. [Google Scholar] [CrossRef]
- Coutinho, E.A.; Kauffman, A.S. The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS). Med. Sci. 2019, 7, 84. [Google Scholar] [CrossRef]
- Ibrahim, Y.F.; Alorabi, M.; Abdelzaher, W.Y.; Toni, N.D.; Thabet, K.; Hegazy, A.; Bahaa, H.A.; Batiha, G.E.; Welson, N.N.; Morsy, M.A.; et al. Diacerein ameliorates letrozole-induced polycystic ovarian syndrome in rats. Biomed. Pharmacother. 2022, 149, 112870. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.Y.; Cho, I.H.; Park, K.S. Therapeutic Effects and Mechanisms of Herbal Medicines for Treating Polycystic Ovary Syndrome: A Review. Front. Pharmacol. 2020, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, H.J.; Pyun, B.J.; Lee, H.W. Licorice ethanol extract improves symptoms of polycytic ovary syndrome in Letrozole-induced female rats. Integr. Med. Res. 2018, 7, 264–270. [Google Scholar] [CrossRef]
- Rajan, R.K.; Kumar, M.S.S.; Balaji, B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm. Biol. 2017, 55, 242–251. [Google Scholar] [CrossRef]
- Ghagane, S.C.; Toragall, M.M.; Akbar, A.A.; Hiremath, M.B. Effect of Aloe vera (Barbadensis Mill) on Letrozole Induced Polycystic Ovarian Syndrome in Swiss Albino Mice. J. Hum. Reprod. Sci. 2022, 15, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Yin, Q.; Xu, X. A Rat Model of Polycystic Ovary Syndrome with Insulin Resistance Induced by Letrozole Combined with High Fat Diet. Med. Sci. Monit. 2020, 26, e922136. [Google Scholar] [CrossRef]
- Shah, M.Z.U.H.; Shrivastava, V.K. Turmeric extract alleviates endocrine-metabolic disturbances in letrozole-induced PCOS by increasing adiponectin circulation: A comparison with Metformin. Metab. Open 2021, 13, 100160. [Google Scholar] [CrossRef] [PubMed]
- Dash, J.R.; Kar, B.; Pattnaik, G. Pharmacological interaction between Antidiabetic drugs and Herbs: An Overview of mechanism of action and clinical implication. Plant Arch. 2020, 20, 3661–3668. [Google Scholar]
- Modi, B.; Kumari Shah, K.; Shrestha, J.; Shrestha, P.; Basnet, A.; Tiwari, I.; Prasad Aryal, S. Morphology, Biological Activity, Chemical Composition, and Medicinal Value of Tinospora cordifolia (Willd.) Miers. Adv. J. Chem.—Sect. B 2020, 2020, 36–54. [Google Scholar]
- Singh, B.; Nathawat, S.; Sharma, R.A. Ethnopharmacological and phytochemical attributes of Indian Tinospora species: A comprehensive review. Arab. J. Chem. 2021, 14, 103381. [Google Scholar] [CrossRef]
- Sharma, R.; Bolleddu, R.; Maji, J.K.; Ruknuddin, G.; Prajapati, P.K. In-Vitro α-amylase, α-glucosidase inhibitory activities and in-vivo anti-hyperglycemic potential of different dosage forms of guduchi (Tinospora cordifolia [Willd.] miers) prepared with ayurvedic bhavana process. Front. Pharmacol. 2021, 12, 642300. [Google Scholar] [CrossRef] [PubMed]
- Pachiappan, S.; Ramalingam, K.; Balasubramanian, A. A review on phytomedicine and their mechanism of action on PCOS. Int. J. Curr. Res. Rev. 2020, 12, 81. [Google Scholar] [CrossRef]
- Harrison, T.N.H.; Chang, R.J. Ovarian response to follicle-stimulating hormone in women with polycystic ovary syndrome is diminished compared to ovulatory controls. Clin. Endocrinol. 2022, 97, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Atashpour, S.; Kargar Jahromi, H.; Kargar Jahromi, Z.; Maleknasab, M. Comparison of the effects of Ginger extract with clomiphene citrate on sex hormones in rats with polycystic ovarian syndrome. Int. J. Reprod. Biomed. 2017, 15, 561–568. [Google Scholar] [CrossRef]
- Jahan, S.; Abid, A.; Khalid, S.; Afsar, T.; Qurat-Ul-Ain.; Shaheen, G.; Almajwal, A.; Razak, S. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: A histological and a biochemical study. J. Ovarian Res. 2018, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Shorakae, S.; Ranasinha, S.; Abell, S.; Lambert, G.; Lambert, E.; de Courten, B.; Teede, H. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin. Endocrinol. 2018, 89, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, Y.; Ma, M.; Li, M. Mechanism of quercetin on the improvement of ovulation disorder and regulation of ovarian CNP/NPR2 in PCOS model rats. J. Formos. Med. Assoc. 2022, 121, 1081–1092. [Google Scholar] [CrossRef]
- Seow, K.M.; Liu, P.S.; Chen, K.H.; Chen, C.W.; Chen, L.K.; Ho, C.H.; Hwang, J.L.; Wang, P.H.; Juan, C.C. Cysteine-Cysteine Motif Chemokine Receptor 5 Expression in Letrozole-Induced Polycystic Ovary Syndrome Mice. Int. J. Mol. Sci. 2021, 23, 134. [Google Scholar] [CrossRef]
- Amanat, S.; Ashkar, F.; Eftekhari, M.H.; Tanideh, N.; Doaei, S.; Gholamalizadeh, M.; Koohpeyma, F.; Mokhtari, M. The effect of genistein on insulin resistance, inflammatory factors, lipid profile, and histopathologic indices in rats with polycystic ovary syndrome. Clin. Exp. Reprod. Med. 2021, 48, 236–244. [Google Scholar] [CrossRef]
- Sharma, R. In Vivo Delivery of Tinospora cordifolia Root Extract Preventing Radiation-Induced Dystrophies in Mice Ovaries. Evid. Based Complement. Alternat. Med. 2015, 2015, 346427. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, R.; Chitme, H.R.; Kukreti, N.; Pant, P.; Abdel-Wahab, B.A.; Khateeb, M.M.; Habeeb, M.S.; Bakir, M.B. Regulation of Insulin Resistance, Lipid Profile and Glucose Metabolism Associated with Polycystic Ovary Syndrome by Tinospora cordifolia. Nutrients 2023, 15, 2238. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15102238
Rani R, Chitme HR, Kukreti N, Pant P, Abdel-Wahab BA, Khateeb MM, Habeeb MS, Bakir MB. Regulation of Insulin Resistance, Lipid Profile and Glucose Metabolism Associated with Polycystic Ovary Syndrome by Tinospora cordifolia. Nutrients. 2023; 15(10):2238. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15102238
Chicago/Turabian StyleRani, Ritu, Havagiray R. Chitme, Neha Kukreti, Pankaj Pant, Basel A. Abdel-Wahab, Masood Medleri Khateeb, Mohammed Shafiuddin Habeeb, and Marwa B. Bakir. 2023. "Regulation of Insulin Resistance, Lipid Profile and Glucose Metabolism Associated with Polycystic Ovary Syndrome by Tinospora cordifolia" Nutrients 15, no. 10: 2238. https://0-doi-org.brum.beds.ac.uk/10.3390/nu15102238