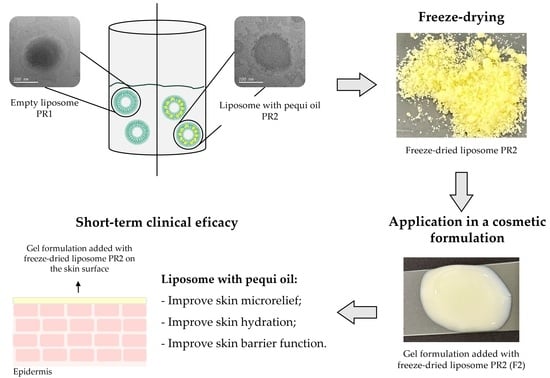
Development and Efficacy Evaluation of Innovative Cosmetic Formulations with Caryocar brasiliense Fruit Pulp Oil Encapsulated in Freeze-Dried Liposomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Liposome Manufacture and Characterization
2.2. Freeze-Dried Liposome Production and Characterization
2.3. Development of Cosmetic Formulations and Stability Testing
2.3.1. Rheology, Texture Profile, and Spreadability Analyses
2.3.2. Short-Term Clinical Efficacy—Immediate Effect Evaluation
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. Liposome Manufacture and Characterization
3.2. Freeze-Dried Liposome Production and Characterization
3.3. Rheology, Texture Profile, and Spreadability Analyss of the Gel-Type Formulations
3.4. Short-Term Clinical Efficacy—Gel-Type Formulations’ Immediate Effect Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, P.M.P.; Arcanjo, D.D.R.; Peron, A.P. Drug development, Brazilian biodiversity and political choices: Where are we heading? J. Toxicol. Environ. Part B 2023, 26, 257–274. [Google Scholar] [CrossRef]
- Silva, G.T.; Di Pietro Fernandes, C.; Hiane, P.A.; Freitas, K.D.C.; Figueiredo, P.S.; Inada, A.C.; Filiú, W.F.; Maldonade, I.R.; Nunes, A.A.; de Oliveira, L.C.S.; et al. Caryocar brasiliense Cambess. Pulp Oil Supplementation Reduces Total Cholesterol, LDL-c, and Non-HDL-c in Animals. Molecules 2020, 25, 4530. [Google Scholar] [CrossRef]
- Pegorin, G.S.A.; Marques, M.O.M.; Mayer, C.R.M.; Santos, L. Development of a Phytocosmetic Enriched with Pequi (Caryocar brasiliense Cambess) Oil. Braz. Arch. Biol. Technol. 2020, 63, e20190478. [Google Scholar] [CrossRef]
- De Figueiredo, P.R.L.; Oliveira, I.B.; Neto, J.B.S.; de Oliveira, J.A.; Ribeiro, L.B.; de Barros Viana, G.S.; Rocha, T.M.; Leal, L.K.A.M.; Felipe, C.F.B.; Coutinho, H.D.M.; et al. Caryocar coriaceum Wittm. (Pequi) fixed oil presents hypolipemic and anti-inflammatory effects in vivo and in vitro. J. Ethnopharmacol. 2016, 191, 87–94. [Google Scholar] [CrossRef]
- Pires, J.; Cargnin, S.T.; Costa, S.A.; Sinhorin, V.D.G.; Damazo, A.S.; Sinhorin, A.P.; de Campos Bicudo, R.; Cavalheiro, L.; de Souza Valladão, D.M.; Pohlmann, A.R.; et al. Healing of dermal wounds property of Caryocar brasiliense oil loaded polymeric lipid-core nanocapsules: Formulation and in vivo evaluation. Eur. J. Pharm. Sci. 2020, 150, 105356. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Silva, N.R.R.D.; Naves, M.M.V. Potential of Whole Pequi (Caryocar spp.) Fruit—Pulp, Almond, Oil, and Shell—As a Medicinal Food. J. Med. Food 2019, 22, 952–962. [Google Scholar] [CrossRef]
- Machado, M.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Vegetable oils oxidation: Mechanisms, consequences and protective strategies. Food Rev. Int. 2022, 39, 4180–4197. [Google Scholar] [CrossRef]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential oils encapsulated in liposomes: A review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Han, J.; Tang, Y. Encapsulating plant ingredients for dermocosmetic application: An updated review of delivery systems and characterization techniques. Int. J. Cosmet. Sci. 2020, 42, 16–28. [Google Scholar] [CrossRef]
- Silva, G.S.; Jange, C.G.; Rocha, J.S.; Chaves, M.A.; Pinho, S.C. Characterisation of curcumin-loaded proliposomes produced by coating of micronised sucrose and hydration of phospholipid powders to obtain multilamellar liposomes. Int. J. Food Sci. 2017, 52, 772–780. [Google Scholar] [CrossRef]
- Lammarı, N.; Louaer, O.; Meniai, A.H.; Fessi, H.; Elaıssarı, A. Plant oils: From chemical composition to encapsulated form use. Int. J. Pharm. 2021, 601, 120538. [Google Scholar] [CrossRef]
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of phenolic compounds with liposomal improvement in the cosmetic industry. Int. J. Pharm. 2021, 593, 120125. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Matricardi, P.; Cencetti, C.; Peris, J.E.; Melis, V.; Carbone, C.; Escribano, E.; Zaru, M.; Fadda, A.M.; Manconi, M. Combination of argan oil and phospholipids for the development of an effective liposome-like formulation able to improve skin hydration and allantoin dermal delivery. Int. J. Pharm. 2016, 505, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, X.; Pan, M.H.; Chiou, Y.S.; Li, Z.; Wei, S.; Yin, X.; Ding, B. The interaction mechanism between liposome and whey protein: Effect of liposomal vesicles concentration. J. Food Sci. 2021, 86, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Bankole, V.O.; Osungunna, M.O.; Souza, C.R.F.; Salvador, S.L.; Oliveira, W.P. Spray-Dried Proliposomes: An innovative method for encapsulation of Rosmarinus officinalis L. polyphenols. AAPS PharmSciTech 2020, 21, 143. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.O.; Maia Campos, P.M.B.G. Application of biophysical and skin imaging techniques to evaluate the film-forming effect of cosmetic formulations. Int. J. Cosmet. Sci. 2019, 41, 579–584. [Google Scholar] [CrossRef]
- Faucheux, E.; Picard, C.; Grisel, M.; Savary, G. Residual film formation after emulsion application: Understanding the role and fate of excipients on skin surface. Int. J. Pharm. 2020, 585, 119453. [Google Scholar] [CrossRef]
- Eudier, F.; Savary, G.; Grisel, M.; Picard, C. Skin surface physico-chemistry: Characteristics, methods of measurement, influencing factors and future developments. Adv. Colloid Interface Sci. 2019, 264, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; de Assunção, R.M.N.; Vieira, A.T.; de Oliveira, M.F.; Batista, A.C.F. Methylic and ethylic biodiesels from pequi oil (Caryocar brasiliense Camb.): Production and thermogravimetric studies. Fuel 2014, 136, 10–18. [Google Scholar] [CrossRef]
- Amazon Oil. Specification of Virgin Pequi Oil (2021). Available online: https://amazonoil.com.br/produtos-da-floresta/pequi-cariocar-brasiliensis/ (accessed on 22 April 2024).
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Tonon, R.V. Secagem por Atomização do Suco de Açai: Influência das Variáveis de Processo, Qualidade e Estabilidade do Produto. Doctoral Thesis, Faculdade de Engenharia de Alimento, Universidade Estadual de Campinas (UNICAMP), Campinas, Brazil, 2009. [Google Scholar]
- Šeremet, D.; Štefančić, M.; Petrović, P.; Kuzmić, S.; Doroci, S.; Mandura Jarić, A.; Cebin, A.V.; Pjanović, R.; Komes, D.; Komes, D. Development, Characterization and Incorporation of Alginate-Plant Protein Covered Liposomes Containing Ground Ivy (Glechoma hederacea L.) Extract into Candies. Foods 2022, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Baldim, I.; Tonani, L.; Von Zeska Kress, M.R.; Oliveira, W.P. Lippia sidoides essential oil encapsulated in lipid nanosystem as an anti-Candida agent. Ind. Crops Prod. 2019, 127, 73–81. [Google Scholar] [CrossRef]
- Meinhardt-Wollweber, M.; Suhr, C.; Kniggendorf, A.-K.; Roth, B. Absorption and resonance Raman characteristics of β-carotene in water-ethanol mixtures, emulsion and hydrogel. AIP Adv. 2018, 8, 055320. [Google Scholar] [CrossRef]
- Assegehegn, G.; Brito-de la Fuente, E.; Franco, J.M.; Gallegos, C. The importance of understanding the freezing step and its impact on freeze-drying process performance. J. Pharm. Sci. 2019, 108, 1378–1395. [Google Scholar] [CrossRef]
- Baldim, I.; Oliveira, A.M.; Souto, E.B.; Oliveira, W.P. Cyclodextrins-in-liposomes: A promising delivery system for Lippia sidoides and Syzygium aromaticum essential oils. Life 2022, 12(1), 95. [Google Scholar] [CrossRef] [PubMed]
- Secolin, V.A.; Souza, C.R.F.; Oliveira, W.P. Spray drying of lipid-based systems loaded with Camellia sinensis polyphenols. J. Liposome Res. 2017, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.M.; Meneguin, A.B.; Fonseca-Santos, B.; de Souza, M.P.C.; Ferreira, L.M.B.; Sabio, R.M.; Chorilli, M.; Gremião, M.P.D. The role of stabilizers and mechanical processes on physico-chemical and anti-inflammatory properties of methotrexate nanosuspensions. J. Drug Deliv. Sci. Technol. 2020, 57, 101638. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C. Precise analysis of nanoparticle size distribution in TEM image. Methods Protoc. 2023, 6, 63. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Kim, B.; Cho, H.E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Sheskey, P.J.; Hancock, B.C.; Moss, G.P.; Goldfarb, D.J. Handbook of Pharmaceutical Excipients, 9th ed.; Pharmaceutical Press: London, UK, 2020. [Google Scholar]
- Kakuda, L.; Campos, P.M.B.G.; Zanin, R.B.; Favaro, L.N. Development of multifunctional sunscreens: Evaluation of physico-mechanical and film-forming properties. Int. J. Pharm. 2023, 635, 122705. [Google Scholar] [CrossRef] [PubMed]
- Calixto, L.S.; Maia Campos, P.M.B.G. Physical-Mechanical characterization of cosmetic formulations and correlation between instrumental measurements and sensorial properties. Int. J. Cosmet. Sci. 2017, 39, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Calixto, L.S.; Infante, V.H.P.; Maia Campos, P.M.B.G. Design and characterization of topical formulations: Correlations between instrumental and sensorial measurements. AAPS PharmSciTech 2018, 19, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.G.A.; Campos, P.M. Correlations between sebaceous glands activity and porphyrins in the oily skin and hair and immediate effects of dermocosmetic formulations. J. Cosm. Dermatol. 2020, 19, 3100–3106. [Google Scholar] [CrossRef] [PubMed]
- Shirata, M.M.F.; Maia Campos, P.M.B.G. Influence of UV filters on the texture profile and efficacy of a cosmetic formulation. Int. J. Cosmet. Sci. 2017, 39, 622–628. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-processing techniques for the improvement of liposome stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Leite, M.G.A.; Maia Campos, P.M. Film-Forming Properties of Topical Formulations for Skin and Hair: In Vivo and In Vitro Studies Using Biophysical and Imaging Techniques. AAPS PharmSciTech 2022, 24, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, A.; Peng, B.; Xu, Y.; Zhang, N. Size-dependent absorption through stratum corneum by drug-loaded liposomes. Pharm. Res. 2021, 38, 1429–1437. [Google Scholar] [CrossRef]
- Try, C.; Moulari, B.; Béduneau, A.; Fantini, O.; Pin, D.; Pellequer, Y.; Lamprecht, A. Size dependent skin penetration of nanoparticles in murine and porcine dermatitis models. Eur. J. Pharm. Biopharm. 2016, 100, 101–108. [Google Scholar] [CrossRef]
- Filon, F.L.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Shin, K.O.; Muraoka, S.; Choi, Y.; Park, J.H.; Park, S.-H.; Hwang, J.-T.; Park, K.; Uchida, Y. The Epidermal Environment’s Influence on the Dermal Environment in Response to External Stress. Skin Pharmacol. Physiol. 2023, 36, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mattos, M.V.C.V.; Michelon, M.; Burkert, J.F.M. Production and stability of food-grade liposomes containing microbial carotenoids from Rhodotorula mucilaginosa. Food Struct. 2022, 33, 100282. [Google Scholar] [CrossRef]
- Agustinisari, I.; Mulia, K.; Nasikin, M. The effect of eugenol and chitosan concentration on the encapsulation of eugenol using whey protein–maltodextrin conjugates. Appl. Sci. 2020, 10, 3205. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. Preparation and characterization of tea tree oil solid liposomes to control brown rot and improve quality in peach fruit. LWT 2022, 162, 113442. [Google Scholar] [CrossRef]
- Boafo, G.F.; Magar, K.T.; Ekpo, M.D.; Qian, W.; Tan, S.; Chen, C. The Role of Cryoprotective Agents in Liposome Stabilization and Preservation. Int. J. Mol. Sci. 2022, 23, 12487. [Google Scholar] [CrossRef]
- Jangle, R.D.; Thorat, B.N. Effect of freeze-thawing study on curcumin liposome for obtaining a better freeze-dried product. Dry. Technol. 2013, 31, 966–974. [Google Scholar] [CrossRef]
- Marchianò, V.; Matos, M.; Serrano, E.; Álvarez, J.R.; Marcet, I.; Blanco-López, M.C.; Gutiérrez, G. Lyophilised nanovesicles loaded with vitamin B12. J. Mol. Liq. 2022, 365, 120129. [Google Scholar] [CrossRef]
- Roy, A.; Dutta, R.; Kundu, N.; Banik, D.; Sarkar, N. A comparative study of the influence of sugars sucrose, Trehalose, and maltose on the hydration and diffusion of DMPC lipid bilayer at complete hydration: Investigation of structural and spectroscopic aspect of lipid–sugar interaction. Langmuir 2016, 32, 5124–5134. [Google Scholar] [CrossRef]
- Varshosaz, J.; Eskandari, S.; Tabbakhian, M. Freeze-drying of nanostructure lipid carriers by different carbohydrate polymers used as cryoprotectants. Carbohydr. Polym. 2012, 88, 1157–1163. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging technologies and coating materials for improved probiotication in food products: A review. Food Bioprocess Technol. 2022, 15, 998–1039. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.A.; Pinho, S.C. Unpurified soybean lecithins impact on the chemistry of proliposomes and liposome dispersions encapsulating vitamin D3. Food Biosci. 2020, 37, 100700. [Google Scholar] [CrossRef]
- Rahman, M.S. Food stability beyond water activity and glass transition: Macro-micro region concept in the state diagram. Int. J. Food Prop. 2009, 12, 726–740. [Google Scholar] [CrossRef]
- Labuza, T.P.; Altunakar, B. Water activity prediction and moisture sorption isotherms. Water Act. Foods Fundam. Appl. 2020, 2, 161–205. [Google Scholar]
- Hinterwirth, H.; Wiedmer, S.K.; Moilanen, M.; Lehner, A.; Allmaier, G.; Waitz, T.; Lindner, W.; Lämmerhofer, M. Comparative method evaluation for size and size-distribution analysis of gold nanoparticles: Other Techniques. J. Sep. Sci. 2013, 36, 2952–2961. [Google Scholar] [CrossRef]
- Yeap, S.P.; Lim, J.; Ngang, H.P.; Ooi, B.S.; Ahmad, A.L. Role of Particle–Particle Interaction Towards Effective Interpretation of Z -Average and Particle Size Distributions from Dynamic Light Scattering (DLS) Analysis. J. Nanosci. Nanotechnol. 2018, 18, 6957–6964. [Google Scholar] [CrossRef] [PubMed]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Lipid Nanoparticles: Production, Characterization and Stability, 1st ed.; Springer International Publishing: New York, NY, USA, 2015; Volume 1, pp. 23–43. [Google Scholar]
- Ito, T.; Sun, L.; Bevan, M.A.; Crooks, R.M. Comparison of nanoparticle size and electrophoretic mobility measurements using a carbon-nanotube-based coulter counter, dynamic light scattering, transmission electron microscopy, and phase analysis light scattering. Langmuir 2004, 20, 6940–6945. [Google Scholar] [CrossRef]
- Mozini, L.M. Brazilian Biodiversity Law: Challenges and Opportunities for Industries and Research Institutions. In Ius Gentium: Comparative Perspectives on Law and Justice; Global Transformations in the Use of Biodiversity for Research and Development; Chege Kamau, E., Ed.; Springer: Cham, Switzerland, 2022; Volume 95. [Google Scholar]
- César, F.C.; Maia Campos, P.M. Influence of vegetable oils in the rheology, texture profile and sensory properties of cosmetic formulations based on organogel. Int. J. Cosmet. Sci. 2020, 42, 494–500. [Google Scholar] [CrossRef]
- Onyeike, E.N.; Acheru, G.N. Chemical composition of selected Nigerian oil seeds and physicochemical properties of the oil extracts. Food Chem. 2002, 77, 431–437. [Google Scholar] [CrossRef]
- Kakuda, L.; Maia Campos, P.M.B.G. Aplicação do Óleo de Pequi para Cuidados da Pele. Cosmet. Toilet. 2023, 35, 16–21. [Google Scholar]
- Ashtiani, H.R.A.; Bishe, P.; Lashgari, N.A.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in cosmetics. J. Skin Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef]
- Sułkowski, W.W.; Pentak, D.; Nowak, K.; Sułkowska, A. The influence of temperature, cholesterol content and pH on liposome stability. J. Mol. Struct. 2005, 744, 737–747. [Google Scholar] [CrossRef]
- Srihera, N.; Li, Y.; Zhang, T.T.; Wang, Y.M.; Yanagita, T.; Waiprib, Y.; Xue, C.H. Preparation and characterization of astaxanthin-loaded liposomes stabilized by sea cucumber sulfated sterols instead of cholesterol. J. Oleo Sci. 2022, 71, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M. New insights on unique features and role of nanostructured materials in cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ann, J.; Othman, N.; Khan, J.; Alolayan, S.O.; Al Thagfan, S.S.; Kaleemullah, M. A Review of Moisturizers; History, Preparation, Characterization and Applications. Cosmetics 2022, 9, 61. [Google Scholar] [CrossRef]








| Phase | Raw Material | Concentration (%) | |
|---|---|---|---|
| PR1 | PR2 | ||
| Oily | Soy Phosphatidylcholine | 5 | 5 |
| Cholesterol | 1 | 1 | |
| Absolute Ethyl Alcohol | 12 | 12 | |
| Pequi Pulp Oil (Caryocar brasiliense Cambess.) | - | 1 | |
| Aqueous | Milli-Q® Water | q.s.p.100 | q.s.p.100 |
| INCI Name * | Concentration % | |
|---|---|---|
| L1 | L2 | |
| Milli-Q® water | q.s.p.100 | q.s.p.100 |
| Ammonium Acryloyldimethyltaurate/VP Copolymer | 0.50 | 0.50 |
| Glycerin | 2.00 | 2.00 |
| Disodium EDTA | 0.05 | 0.05 |
| Xylityl Sesquicaprylate | 0.80 | 0.80 |
| Freeze-dried liposome PR1 (empty) | 4.00 | - |
| Freeze-dried liposome PR2 (1% Pequi oil—Caryocar brasiliense Cambess pulp oil) | - | 4.00 |
| Time (Days) | Liposome Pre-Formulations/Storage Temperature (°C) | |||||
|---|---|---|---|---|---|---|
| PR1 T5 | PR1 T25 | PR1 T37 | PR2 T5 | PR2 T25 | PR2 T37 | |
| T0 | −28.43 | −28.43 | −28.43 | −29.87 | −29.87 | −29.87 |
| T7 | −35.10 | −31.13 | −31.47 | −33.03 | −34.40 | −33.73 |
| T14 | −34.07 | −31.00 | −29.60 | −30.63 | −33.60 | −30.37 |
| T28 | −31.10 | −33.47 | −29.60 | −30.13 | −36.73 | −30.20 |
| T49 | −34.33 | −31.03 | −29.20 | −31.93 | −30.07 | −30.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakuda, L.; Maia Campos, P.M.B.G.; Oliveira, W.P. Development and Efficacy Evaluation of Innovative Cosmetic Formulations with Caryocar brasiliense Fruit Pulp Oil Encapsulated in Freeze-Dried Liposomes. Pharmaceutics 2024, 16, 595. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050595
Kakuda L, Maia Campos PMBG, Oliveira WP. Development and Efficacy Evaluation of Innovative Cosmetic Formulations with Caryocar brasiliense Fruit Pulp Oil Encapsulated in Freeze-Dried Liposomes. Pharmaceutics. 2024; 16(5):595. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050595
Chicago/Turabian StyleKakuda, Letícia, Patrícia M. B. G. Maia Campos, and Wanderley P. Oliveira. 2024. "Development and Efficacy Evaluation of Innovative Cosmetic Formulations with Caryocar brasiliense Fruit Pulp Oil Encapsulated in Freeze-Dried Liposomes" Pharmaceutics 16, no. 5: 595. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics16050595