Relationships between Interleukin 18 -607 C/A and -137 G/C, Osteopontin -9250 C/T Genetic Polymorphisms and Systemic Inflammatory Response Syndrome in Coronary Artery Bypass Graft Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
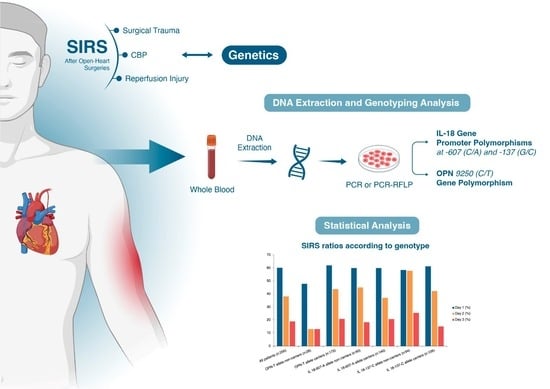
2.2. DNA Extraction and Genotyping Analysis
2.3. IL-18 Gene Promoter Polymorphisms
2.4. OPN 9250 CT Gene Polymorphism
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zakkar, M.; Ascione, R.; James, A.F.; Angelini, G.D.; Suleiman, M.S. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Ther. 2015, 1i54, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Sutherland, A.M.; Russell, J.A.; Lichtenstein, S.V.; Walley, K.R. Novel polymorphism of interleukin-18 associated with greater inflammation after cardiac surgery. Crit. Care 2009, 13, R9. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.E.A.; de Sousa, E.L.H.; de Oliveira Rodrigues, R.; de Almeida Viana, G.; Gadelha, D.D.; de Carvalho, M.M.D.; Queiroz, M.G.R. Interleukin-18 promoter −137 G/C polymorphism (rs187238) is associated with biochemical markers of renal function and cardiovascular disease in type 2 diabetes patients. Clin. Biochem. 2020, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Arapi, B.; Bayoğlu, B.; Cengiz, M.; Dirican, A.; Deser, S.B.; Junusbekov, Y.; Arslan, C. Increased Expression of Interleukin-18 mRNA is Associated with Carotid Artery Stenosis. Balkan Med. J. 2018, 35, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Karadag, M.E. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Uede, T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011, 61, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, J.; Kon, S.; Matsui, Y.; Uede, T. Osteopontin; as a target molecule for the treatment of inflammatory diseases. Curr. Drug Targets. 2010, 11, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Sano, M. Osteopontin in Cardiovascular Diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- Vaschetto, R.; Nicola, S.; Olivieri, C.; Boggio, E.; Piccolella, F.; Mesturini, R.; Chiocchetti, A. Serum levels of osteopontin are increased in SIRS and sepsis. Intensive Care Med. 2008, 34, 2176–2184. [Google Scholar] [CrossRef]
- Kocabaş, N.A.; Karahalil, B. XRCC1 Arg399Gln genetic polymorphism in a Turkish population. Int. J. Toxicol. 2006, 25, 419–422. [Google Scholar] [CrossRef]
- Wei, Y.S.; Lan, Y.; Liu, Y.G.; Tang, H.; Tang, R.G.; Wang, J.C. Interleukin-18 gene promoter polymorphisms and the risk of esophageal squamous cell carcinoma. Acta Oncologica. 2007, 46, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Kerr, J.M.; Termine, J.D.; Wewer, U.M.; Wang, M.G.; McBride, O.W.; Fisher, L.W. cDNA cloning mRNA distribution and heterogeneity chromosomal location and RFLP analysis of human osteopontin (OPN). Genomics 1990, 7, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Tanaka, A.; Miyakawa, H.; Kawashima, Y.; Kawaguchi, N.; Matsushita, M.; Gershwin, M.E. Eta-1/osteopontin genetic polymorphism and primary biliary cirrhosis. Hepatol. Res. 2003, 26, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.P.; Jie, B.A.I.; Jun, L.Ü.; Liang, Y.Y.; Li, J.G.; Lai, D.Y.; Huang, H.H. Osteopontin gene polymorphism in association with systemic lupus erythematosus in Chinese patients. Chin. Med. J. 2007, 120, 2124–2128. [Google Scholar] [CrossRef]
- Salimi, S.; Noora, M.; Nabizadeh, S.; Rezaei, M.; Shahraki, H.; Milad, M.K.; Sandoughi, M. Association of the osteopontin rs 1126616 polymorphism and a higher serum osteopontin level with lupus nephritis. Biomed. Reports. 2016, 4, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-López, P.; Carretero, R.; Vazquez, F.; Martin, J.; Sánchez, E.; Tallada, M.; Ruiz-Cabello, F. Impact of interleukin-18 polymorphisms -607 and -137 on clinical characteristics of renal cell carcinoma patients. Hum. Immunol. 2010, 71, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.A.; Severson, A.; Edwards, W.D.; Ingram, R.T. Diffuse calcification in human coronary arteries association of osteopontin with atherosclerosis. J. Clin. Invest. 1994, 94, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.H.; Sanchez-Ross, M.; Kaluski, E.; Klapholz, M. Osteopontin in cardiovascular disease: A potential therapeutic target. Cardiol. Rev. 2010, 18, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Sauaia, A.; Moore, F.A.; Moore, E.E. Postinjury inflammation and organ dysfunction. Crit. Care Clin. 2017, 33, 167–191. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. Am. Coll. Chest Physicians/Soc. Crit. Care Medicine. Chest. 1992, 101, 1644–1655. [Google Scholar]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Paparella, D. Prevalence and clinical impact of systemic inflammatory reaction after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, N.S.; Finney, S.J.; Gordon, S.E.; Quinlan, G.J.; Etans, T.W. Modified criteria for the systemic inflammatory response syndrome improves their utility following cardiac surgery. Chest 2014, 145, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Rogall, R.; Rabenstein, M.; Vay, S.; Bach, A.; Pikhovych, A.; Baermann, J.; Rueger, M.A. Bioluminescence imaging visualizes osteopontin-induced neurogenesis and neuroblast migration in the mouse brain after stroke. Stem. Cell Res. Therapy. 2018, 9, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hui, T.; Matsui, A.; Allahem, Z.; Johnston, C.D.; Ruiz-Torruella, M.; Rittling, S.R. Modulation of infection-mediated migration of neutrophils and CXCR2 trafficking by osteopontin. Immunology. 2017, 150, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Barreno, R.X.; Richards, J.B.; Schneider, D.J.; Cromar, K.R.; Nadas, A.J.; Hernandez, C.B.; Johnston, R.A. Endogenous osteopontin promotes ozone-induced neutrophil recruitment to the lungs and airway hyperresponsiveness to metacholine. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L118–L129. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, G.J.; Hoogendijk, A.J.; Schouten, M.; Hommes, T.J.; de Vos, A.F.; Florquin, S.; van der Poll, T. Osteopontin impairs host defense during pneumococcal pneumonia. J. Infect. Dis. 2011, 203, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K. Unique action of interleukin-18 on T cells and other immune cells. Cells Front. Immunol. 2018, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in health and disease. Int. J. MolSci. 2019, 20, 649. [Google Scholar] [CrossRef] [PubMed]
- Giedraitis, V.; He, B.; Huang, W.X.; Hillert, J. Cloning and mutation analysis of the human IL-18 promoter: A possible role of polymorphisms in expression regulation. J. Neuroimmunol. 2001, 112, 146–152. [Google Scholar] [CrossRef]
- Sablotzki, A.; Friedrich, I.; Mühling, J.; Dehne, M.G.; Spillner, J.; Silber, R.E.; Czeslik, E. The systemic inflammatory response syndrome following cardiac surgery: Different expression of proinflammatory cytokines and procalcitonin in patients with and without multiorgan dysfunctions. Perfusion 2002, 17, 103–109. [Google Scholar] [CrossRef]
- Yamada, T.; Aoyama-Ishikawa, M.; Yamashita, H.; Fujiwara, M.; Usami, M.; Ueda, T.; Kotani, J. IL 18 production and IL 18 promoter polymorphisms correlate with mortality in ICU patients. In Vivo 2014, 28, 391–396. [Google Scholar] [PubMed]
- Kretowski, A.; Mironczuk, K.; Karpinska, A.; Bojaryn, U.; Kinalski, M.; Puchalski, Z.; Kinalska, I. Interleukin-18 promoter polymorphisms in type 1 diabetes. Diabetes 2002, 51, 3347–3349. [Google Scholar] [CrossRef] [PubMed]
- Mitrokhin, V.; Nikitin, A.; Brovkina, O.; Khodyrev, D.; Zotov, A.; Vachrushev, N.; Kamkin, A. Association between IL-18/18R gene polymorphisms and coronary artery disease: Influence of IL-18/18R genetic variants on cytokine expression. J. Inflamm. Res. 2018, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients | OPN-T Allele Non-Carriers | OPN-T Allele Carriers | p Value | IL 18-607-A Allele Non-Carriers | IL 18-607-A Allele Carriers | p Value | IL 18-137-C Allele Non-Carriers | IL 18-137-C Allele Carriers | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | 200 | 28 | 172 | NA | 60 | 140 | NA | 94 | 106 | NA |
| Gender (F/M) | 36/164 | 6/22 | 30/143 | 0.188 | 12/48 | 24/116 | 0.360 | 17/77 | 18/88 | 0.520 |
| Age (Years) | 59.6 ± 8.9 | 60 ± 7.2 | 59.7 ± 9.1 | 0.876 | 59 ± 8.1 | 60 ± 9.1 | 0.286 | 56.9 ± 9.1 | 59.6 ± 8.6 | 0.352 |
| Heights (cm) | 166.8 ± 7 | 164.8 ± 8.2 | 167.1 ± 7.2 | 0.150 | 167.1 ± 6.7 | 166.7 ± 7.2 | 0.416 | 166.2 ± 6.8 | 167.4 ± 7.2 | 0.385 |
| Weights (kg) | 76.6 ± 11.8 | 74.6 ± 12.4 | 77.1 ± 11.8 | 0.366 | 76 ± 11.6 | 77 ± 12 | 0.975 | 76.1 ± 12.1 | 77.3 ± 11.7 | 0.308 |
| BMI (kg/m2) | 27.5 ± 4 | 27.3 ± 4.2 | 27.6 ± 4 | 0.799 | 27.2 ± 4.2 | 27.6 ± 3.9 | 0.333 | 27.5 ± 4 | 27.5 ± 4 | 0.351 |
| Pre-existing conditions, n (%) | ||||||||||
| Smoking habits | 66 (33) | 13 (46.4) | 53 (30.8) | 0.055 | 24 (40) | 42 (30) | 0.100 | 36 (38.2) | 30 (28.3) | 0.183 |
| Diabetes | 87 (43.5) | 13 (46.4) | 74 (43) | 0.579 | 23 (38.3) | 65 (46.4) | 0.199 | 40 (44.6) | 47 (44.3) | 0.401 |
| Hypertension | 128 (64) | 18 (64.2) | 110(63.9) | 0.504 | 39 (65) | 89 (63.5) | 0.520 | 61 (64.8) | 67 (64.1) | 0.521 |
| Hyperlipidemia | 93 (46.5) | 13 (46.4) | 80 (46.5) | 0.552 | 28 (46.6) | 65 (46.4) | 0.542 | 43 (46.2) | 50 (47.1) | 0.540 |
| COPD | 36 (18) | 7 (25) | 29 (16.8) | 0.246 | 16(26.6) | 20 (14.2) | 0.087 | 22 (23.4) | 14 (13.2) | 0.121 |
| Neurologic disorder | 22 (11) | 3 (10.7) | 19 (11) | 0.521 | 6 (10) | 16 (11.4) | 0.528 | 9 (9.5 | 13 (13.7) | 0.318 |
| Medications, n (%) | ||||||||||
| ASA | 89 (44.5) | 11 (39.2) | 74 (43) | 0.435 | 28 (46.6) | 59 (42.1) | 0.407 | 40 (42.5) | 49 (46.2) | 0.433 |
| Clopidogrel | 24 (12) | 4 (14.2) | 18 (10.4) | 0.483 | 8 (13.3) | 13 (9.2) | 0.279 | 12 (12.7) | 12 (11.3) | 0.310 |
| Statins | 81 (40.5) | 13 (46.4) | 67 (38.9) | 0.247 | 23 (38.3) | 57 (40.7) | 0.433 | 39 (41.4) | 42 (39.6) | 0.462 |
| Beta-blockers | 85 (42.5) | 10 (35.7) | 73 (42.4) | 0.325 | 25 (41.6) | 58 (41.4) | 0.565 | 34 (36.1) | 51 (48.1) | 0.108 |
| ACE inhibitors | 72 (36) | 13 (46.4) | 63 (36.6) | 0.171 | 20 (33.3) | 52 (38) | 0.408 | 32 (34) | 40 (37.7) | 0.390 |
| Ca channel blockers | 49 (24.5) | 4 (14.2) | 43 (25) | 0.144 | 18 (30) | 30 (21.4) | 0.173 | 25 (26.5) | 24 (22.6) | 0.281 |
| Gene Polymorphism | Study Population n (%) | Healthy Caucasian n (%) | p Value |
|---|---|---|---|
| IL 18 -137 G/C | |||
| GG | 94 (47) | 251(50) | 0.313 |
| GC | 98 (49) | 220 (43.8) | |
| CC | 8 (4) | 31 (6.2) | |
| IL 18 -607 C/A | |||
| CC | 60 (30) | 166 (33.2) | 0.020 |
| CA | 128 (64) | 261 (52.2) | |
| AA | 12 (6) | 73 (14.6) | |
| OPN -9250 C/T | |||
| CC | 28 (14) | 138 (76.7) | 0.001 |
| CT | 137 (68.5) | 40 (22.2) |
| SIRS | All Patients (n = 200) | OPN-T Allele Non-Carriers (n = 28) | OPN-T Allele Carriers (n = 172) | p Value | IL 18-607-A Allele Non-Carriers (n = 60) | IL 18-607-A Allele Carriers (n = 140) | p Value | IL 18-137-C Allele Non-Carriers (n = 94) | IL 18-137-C Allele Carriers (n = 106) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 (%) | 60.2 | 47.8 | 62 | 0.145 | 60 | 60 | 0.520 | 58.5 | 61.3 | 0.423 |
| Day 2 (%) | 38.1 | 13 | 43.7 | 0.004 * | 45 | 37.1 | 0.226 | 57.8 | 42.2 | 0.025 * |
| Day 3 (%) | 18.9 | 13 | 20.8 | 0.293 | 18.3 | 20.7 | 0.489 | 25.5 | 15 | 0.068 |
| All Patients | OPN-T Allele Non-Carriers | OPN-T Allele Carriers | p Value | IL 18-607-A Allele Non-Carriers | IL 18-607-A Allele Carriers | p Value | IL 18-137-C Allele Non-Carriers | IL 18-137-C Allele Carriers | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| EF (%) | 51.6 ± 10.6 | 50.8 ± 13.3 | 51.5 ± 10.3 | 0.507 | 51 ± 12.4 | 51.4 ± 9.9 | 0.071 | 49.7 ± 12 | 52.8 ± 9.3 | 0.173 |
| X-Clamping time (min) | 63.4 ± 25 | 65.8 ± 26.9 | 63.2 ± 24.9 | 0.487 | 67.3 ± 29.3 | 62.3 ± 23.1 | 0.027 * | 66.5 ± 27.3 | 61.5 ± 22.9 | 0.134 |
| CPB time (min) | 98.3 ± 35 | 104.8 ± 41.4 | 97.4 ± 33.9 | 0.227 | 101.5 ± 41.5 | 97.7 ± 31.9 | 0.012 * | 101.8 ± 38.3 | 96.2 ± 31.8 | 0.127 |
| Operation time (min) | 261.7 ± 61.9 | 266 ± 90.6 | 259 ± 56.5 | 0.220 | 271.4 ± 63.6 | 256.7 ± 61.8 | 0.213 | 270.2 ± 67.1 | 252.9 ± 57.4 | 0.464 |
| Intubation time (h) | 13.3 ± 14.1 | 10.7 ± 4.8 | 13.3 ± 14.7 | 0.322 | 11.8 ± 8.2 | 13.5 ± 15.4 | 0.267 | 12.3 ± 7.6 | 13.5 ± 17.3 | 0.228 |
| ICU stay (day) | 2.1 ± 5.1 | 1.7 ± 2.9 | 2.1 ± 5.4 | 0.556 | 2.6 ± 7.1 | 1.9 ± 4.1 | 0.106 | 3.1 ± 7.4 | 1.2 ± 0.9 | 0.001 * |
| Hospital stay (day) | 7.8 ± 6.4 | 7.6 ± 4.1 | 7.7 ± 6.6 | 0.669 | 8.4 ± 8.5 | 7.6 ± 5.4 | 0.248 | 8.7 ± 8.3 | 7 ± 4.1 | 0.003 * |
| Preoperative WBC (×109/L) | 8.1 ± 1.9 | 7.4 ± 1.7 | 8.1 ± 1.9 | 0.091 | 7.8 ± 1.9 | 8.1 ± 1.9 | 0.315 | 8.0 ± 1.8 | 8.0 ± 2 | 0.865 |
| Postoperative 1st day WBC (×109/L) | 11.5 ± 3.4 | 10.4 ± 2.9 | 11.8 ± 3.4 | 0.077 | 12.1 ± 3.9 | 11.3 ± 3.2 | 0.132 | 11.6 ± 3.5 | 11.4 ± 3.4 | 0.755 |
| Postoperative 2nd day WBC (×109/L) | 12.3 ± 3.8 | 10.5 ± 2.4 | 12.7 ± 4 | 0.015 * | 12.3 ± 3.9 | 12.3 ± 3.8 | 0.961 | 12.4 ± 3.3 | 12.3 ± 4.3 | 0.937 |
| Postoperative 3rd day WBC (×109/L) | 11.3 ± 4.1 | 9.1 ± 4.7 | 11.8 ± 4 | 0.035 * | 10.8 ± 3.3 | 11.6 ± 4.6 | 0.399 | 10.9 ± 3.8 | 11.8 ± 4.5 | 0.327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köse, S.K.; Karahilal, B.; Engin, B.; Aydoğdu, G.; Yağar, S.; Orhan, K. Relationships between Interleukin 18 -607 C/A and -137 G/C, Osteopontin -9250 C/T Genetic Polymorphisms and Systemic Inflammatory Response Syndrome in Coronary Artery Bypass Graft Surgery. Medicina 2024, 60, 724. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina60050724
Köse SK, Karahilal B, Engin B, Aydoğdu G, Yağar S, Orhan K. Relationships between Interleukin 18 -607 C/A and -137 G/C, Osteopontin -9250 C/T Genetic Polymorphisms and Systemic Inflammatory Response Syndrome in Coronary Artery Bypass Graft Surgery. Medicina. 2024; 60(5):724. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina60050724
Chicago/Turabian StyleKöse, Serdal Kenan, Bensu Karahilal, Başak Engin, Gülçin Aydoğdu, Seyhan Yağar, and Kaan Orhan. 2024. "Relationships between Interleukin 18 -607 C/A and -137 G/C, Osteopontin -9250 C/T Genetic Polymorphisms and Systemic Inflammatory Response Syndrome in Coronary Artery Bypass Graft Surgery" Medicina 60, no. 5: 724. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina60050724