Alginate Films Enriched in Raspberry and/or Black Currant Seed Oils as Active Food Packaging
Abstract
:1. Introduction
2. Results
2.1. ATR-FTIR Spectroscopy Results
2.2. Thickness, Opacity, Moisture Content, and Water Vapor Transmission Rate (WVTR) Results
2.3. Mechanical Properties
2.4. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of Physicochemical Parameters of the Tested Oils
4.3. Determination of Sodium Alginate Molecular Weight
4.4. Film Preparation
4.5. ATR-FTIR Spectroscopy
4.6. UV-VIS Spectroscopy
4.7. The Tensile Tests
4.8. Moisture Content
4.9. Water Vapor Transmission Rate (WVTR)
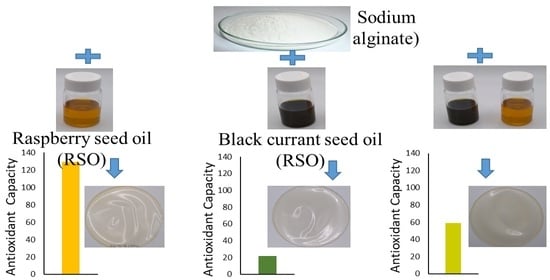
4.10. Antioxidant Capacity
4.11. Statistical Analysis
4.12. Antibacterial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.D.; Flores, S.K.; Marangoni, A.G.; Gerschenson, L.N.; Rojas, A.M. Development of a High Methoxyl Pectin Edible Film for Retention of L-(+)-Ascorbic Acid. J. Agric. Food Chem. 2009, 57, 6844–6855. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Trafiałek, J.; Kolanowski, W. Edible Packaging: A Technological Update for the Sustainable Future of the Food Industry. Appl. Sci. 2023, 13, 8234. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of Glycerol Plasticizer Loading on the Physical, Mechanical, Thermal, and Barrier Properties of Arrowroot (Maranta arundinacea) Starch Biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Dobrucka, R.; Przekop, R. New Perspectives in Active and Intelligent Food Packaging. J. Food Process. Preserv. 2019, 43, e14194. [Google Scholar] [CrossRef]
- Makała, H. Active And Intelligent Food Packaging Review Paper, Part 1. Packag. Rev. 2023, 1, 17–23. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Islam, M.; Grygier, A.; Tomaszewska-Gras, J. Thermal and Spectroscopic Profiles Variation of Cold-Pressed Raspberry Seed Oil Studied by DSC, UV/VIS, and FTIR Techniques. J. Food Compos. Anal. 2023, 124, 105723. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Orzołek, M. The Correlation between Nutritional and Health Potential and Antioxidant Properties of Raw Edible Oils from Cultivated and Wild Plants. Int. J. Food Sci. Technol. 2023, 58, 676–685. [Google Scholar] [CrossRef]
- Dave Oomah, B.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of Raspberry (Rubus idaeus L.) Seed Oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Andlid, T.; Lundin, L.; Ahrné, L.; Alminger, M. Supercritical Fluid Extraction of Berry Seeds: Chemical Composition and Antioxidant Activity. J. Food Qual. 2018, 2018, 6046074. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Schieber, A.; Kolodziejczyk, P. Characterization of Canadian Black Currant (Ribes nigrum L.) Seed Oils and Residues. J. Agric. Food Chem. 2009, 57, 11528–11536. [Google Scholar] [CrossRef] [PubMed]
- Poiana, M.A.; Alexa, E.; Munteanu, M.F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR Spectroscopy to Detect the Changes in Extra Virgin Olive Oil by Adulteration with Soybean Oil and High Temperature Heat Treatment. Open Chem. 2015, 13, 689–698. [Google Scholar] [CrossRef]
- Dong, X.; Li, Q.; Sun, D.; Chen, X.; Yu, X. Direct FTIR Analysis of Free Fatty Acids in Edible Oils Using Disposable Polyethylene Films. Food Anal. Methods 2015, 8, 857–863. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Infrared Spectroscopy in the Study of Edible Oils and Fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Sota-Uba, I.; Bamidele, M.; Moulton, J.; Booksh, K.; Lavine, B.K. Authentication of Edible Oils Using Fourier Transform Infrared Spectroscopy and Pattern Recognition Methods. Chemom. Intell. Lab. Syst. 2021, 210, 104251. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier Transform-Infrared Spectroscopy to Edible Oils. Anal. Chim. Acta 2006, 573–574, 459–465. [Google Scholar] [CrossRef]
- Szafran, M.D.-S.Z. Organic Compounds Structure Deteremination with Spectroscopic Methods; PWN: Warsaw, Poland, 1988. [Google Scholar]
- Rajca, A. Spectroscopic Methods and Their Applications to Organic Compounds Identification; WNT: Warsaw, Poland, 1995. [Google Scholar]
- Mutlu, N. Effects of Grape Seed Oil Nanoemulsion on Physicochemical and Antibacterial Properties of Gelatin-sodium Alginate Film Blends. Int. J. Biol. Macromol. 2023, 237, 124207. [Google Scholar] [CrossRef]
- Nehchiri, N.; Amiri, S.; Radi, M. Improving the Water Barrier Properties of Alginate Packaging Films by Submicron Coating with Drying Linseed Oil. Packag. Technol. Sci. 2021, 34, 283–295. [Google Scholar] [CrossRef]
- Kadzińska, J.; Bryś, J.; Ostrowska-Ligęza, E.; Estéve, M.; Janowicz, M. Influence of Vegetable Oils Addition on the Selected Physical Properties of Apple–Sodium Alginate Edible Films. Polym. Bull. 2020, 77, 883–900. [Google Scholar] [CrossRef]
- Trela, A.; Szymańska, R. Less Widespread Plant Oils as a Good Source of Vitamin E. Food Chem. 2019, 296, 160–166. [Google Scholar] [CrossRef]
- Kowalonek, J.; Stachowiak, N.; Bolczak, K.; Richert, A. Physicochemical and Antibacterial Properties of Alginate Films Containing Tansy (Tanacetum vulgare L.) Essential Oil. Polymers 2023, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, M.S.; Salama, H.E.; Sabaa, M.W. Biobased Alginate/Castor Oil Edible Films for Active Food Packaging. LWT 2018, 96, 455–460. [Google Scholar] [CrossRef]
- Zhang, F.; Bai, X.; Wei, G.; Wang, G.; Shi, Z.; Jun, C. Effects of Virgin Coconut Oil on the Physicochemical, Morphological and Antibacterial Properties of Potato Starch-Based Biodegradable Films. Int. J. Food Sci. Technol. 2020, 55, 192–200. [Google Scholar] [CrossRef]
- Colivet, J.; Garcia, V.A.d.S.; Lourenço, R.V.; Yoshida, C.M.P.; Oliveira, A.L.d.; Vanin, F.M.; Carvalho, R.A.d. Characterization of Films Produced with Cross-Linked Cassava Starch and Emulsions of Watermelon Seed Oils. Foods 2022, 11, 3803. [Google Scholar] [CrossRef] [PubMed]
- Khah, M.D.; Ghanbarzadeh, B.; Roufegarinejad Nezhad, L.; Ostadrahimi, A. Effects of Virgin Olive Oil and Grape Seed Oil on Physicochemical and Antimicrobial Properties of Pectin-Gelatin Blend Emulsified Films. Int. J. Biol. Macromol. 2021, 171, 262–274. [Google Scholar] [CrossRef]
- PN-ISO 660:1998; Vegetable and Animal Oils and Fats. Determination of Acid Number and Acidity. PKN: Warsaw, Poland, 1998.
- Hopper, T.H.; Nesbitt, K.L. Relation between the Refractive Index and Iodine Number of Raw Linseed Oil. Oil Soap 1937, 14, 34–36. [Google Scholar] [CrossRef]
- Hunt, W.H.; Neustadt, M.H.; Shurkus, A.A.; Zeleny, L. A Simple Iodine-Number Refractometer for Testing Flaxseed and Soybeans. J. Am. Oil Chem. Soc. 1951, 28, 5–8. [Google Scholar] [CrossRef]
- 34. PN-ISO 3960:1996; Determination of Peroxide Value. PKN: Warsaw, Poland, 1996.
- Neagu, A.-A.; Niţa, I.; Botez, E.; Geaca, S. A Physico-Chemical Study for Some Edible Oils Properties. Analele Univ. “Ovidius” Constanta—Ser. Chim. 2014, 24, 121–126. [Google Scholar] [CrossRef]
- ISO 20645:2006; Flat Products—Determination of Antimicrobial Activity—Diffusion Method on Agar Plate. ISO: Geneva, Switzerland, 2006.
- Tymczewska, A.; Furtado, B.U.; Nowaczyk, J.; Hrynkiewicz, K.; Szydłowska-Czerniak, A. Functional Properties of Gelatin/Polyvinyl Alcohol Films Containing Black Cumin Cake Extract and Zinc Oxide Nanoparticles Produced via Casting Technique. Int. J. Mol. Sci. 2022, 23, 2734. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A. Antioxidant Capacity of Rapeseed Extracts Obtained by Conventional and Ultrasound-Assisted Extraction. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 2011–2019. [Google Scholar] [CrossRef] [PubMed]





| Frequency (cm−1) | Vibrations |
|---|---|
| 3008 | =C−H stretching in the cis isomers |
| 2923 | −C−H asymmetric stretching in CH2 |
| 2853 | −C−H symmetric stretching in CH2 |
| 1743 | −C=O stretching in esters |
| 1705 | −C=O stretching in free fatty acids |
| 1650 | −HC=CH− stretching in cis isomer |
| 1463 | −C−H scissoring in CH2, −CH3 |
| 1377 | −C−H scissoring CH3 |
| 1236 | −C−O stretching, −C−H bending |
| 1160 | −C−O stretching, −C−H bending |
| 1098 | −C−O stretching |
| 964 | −HC=CH− bending in trans isomers |
| 914 | −HC=CH− bending in cis isomers |
| 721 | −HC=CH− bending in cis isomers, C−H rocking −(CH2)n− |
| Sample | Thickness (mm) | Opacity, A600/Thickness | Mc (%) | WVTR (g/(m2 × 24 h)) | AC (μmol Trolox/100 g) |
|---|---|---|---|---|---|
| Alg + G | 0.116 ± 0.009 a | 0.436 | 37.96 ± 3.54 a | 296 ± 14 a | 0 a |
| Alg + G+ RSO (25%) | 0.166 ± 0.014 b | 1.989 | 32.43 ± 0.96 a | 265 ± 2 b | 64.71 ± 22.43 b |
| Alg + G+ RSO (50%) | 0.184 ± 0.012 c | 2.125 | 27.94 ± 1.81 ab | 245 ± 9 b | 128.68 ± 35.92 c |
| Alg + G + BCSO (25%) | 0.134 ± 0.008 d | 1.011 | 26.96 ± 0.15 b | 268 ± 3 b | 6.78 ± 2.30 a |
| Alg + G + BCSO (50%) | 0.177 ± 0.014 c | 1.645 | 23.40 ± 0.59 b | 263 ± 1 b | 21.43 ± 3.46 ab |
| Alg + G + (RSO + BCSO) (25%) | 0.136 ± 0.001 d | 2.169 | 27.41 ± 1.44 ab | 290 ± 5 a | 55.47 ± 0.21 b |
| Alg + G + (RSO + BCSO) (50%) | 0.161 ± 0.008 b | 2.466 | 22.87 ± 1.39 b | 253 ± 13 b | 59.05 ± 15.22 b |
| Sample | Young’s Modulus, E (MPa) | Stress at the Break, σ (MPa) | Strain at the Break, ε (%) |
|---|---|---|---|
| Alg + G | 22.55 ± 3.26 a | 7.41 ± 1.18 a | 34.75 ± 2.07 a |
| Alg + G + RSO (25%) | 14.68 ± 1.21 b | 6.08 ± 1.46 a | 41.36 ± 7.83 a |
| Alg + G + RSO (50%) | 11.49 ± 0.69 b | 5.57 ± 0.85 a | 45.14 ± 4.23 ab |
| Alg + G + BCSO (25%) | 9.12 ± 0.55 cb | 5.21 ± 0.66 a | 43.84 ± 2.78 ab |
| Alg + G + BCSO (50%) | 7.06 ± 0.67 c | 5.24 ± 0.81 a | 49.8 ± 2.15 b |
| Alg + G + (RSO + BCSO) (25%) | 12.58 ± 0.56 b | 7.15 ± 0.51 a | 45.36 ± 2.35 ab |
| Alg + G + (RSO + BCSO) (50%) | 9.8 ± 0.61 cb | 4.56 ± 0.55 b | 37.79 ± 2.88 a |
| Oil Type | AV (mg KOH/1 g Sample) | IV (g I2/100 g Sample) | PV (mEq O2/kg Sample) | nD25 |
|---|---|---|---|---|
| RSO | 4.03 ± 0.10 a | 126.5 ± 4.31 a | 20.89 ± 1.31 a | 1.4724 ± 0.0005 |
| BCSO | 2.65 ± 0.09 b | 122.21 ± 3.97 a | 21.28 ± 0.95 a | 1.4719 ± 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalonek, J.; Łukomska, B.; Łukomska, O.; Stachowiak-Trojanowska, N. Alginate Films Enriched in Raspberry and/or Black Currant Seed Oils as Active Food Packaging. Molecules 2024, 29, 2012. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29092012
Kowalonek J, Łukomska B, Łukomska O, Stachowiak-Trojanowska N. Alginate Films Enriched in Raspberry and/or Black Currant Seed Oils as Active Food Packaging. Molecules. 2024; 29(9):2012. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29092012
Chicago/Turabian StyleKowalonek, Jolanta, Bogna Łukomska, Olga Łukomska, and Natalia Stachowiak-Trojanowska. 2024. "Alginate Films Enriched in Raspberry and/or Black Currant Seed Oils as Active Food Packaging" Molecules 29, no. 9: 2012. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules29092012