Sensitive Silver-Enhanced Microplate Apta-Enzyme Assay of Sb3+ Ions in Drinking and Natural Waters
Abstract
:1. Introduction
2. Results and Discussion
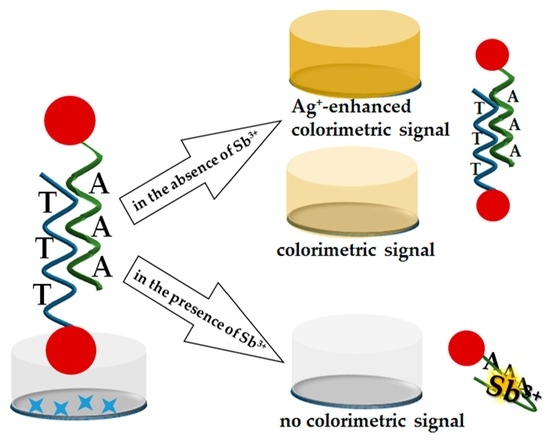
2.1. Principle of Sb3+ Detection
2.2. Characterization of the Apta-Assay Components via CD Spectroscopy
2.3. Optimization of the Microplate Apta-Enzyme Assay Conditions
2.4. Analytical Performance of the Microplate Apta-Enzyme Assay of Sb3+
2.5. Ag+-Enhanced Microplate Apta-Enzyme Assay for Sb3+ Detection
2.6. Selectivity of Ag+-Enhanced Microplate Apta-Enzyme Assay
2.7. Water Sample Analysis
2.8. Comparison with Other Methods
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Formation of A10/T10 and A10/Ag+/T10 Complexes
3.3. Circular Dichroism Measurement
3.4. Microplate Apta-Enzyme Assay
3.5. Preparation and Analysis of Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- He, M.; Wang, N.; Long, X.; Zhang, C.; Ma, C.; Zhong, Q.; Wang, A.; Wang, Y.; Pervaiz, A.; Shan, J. Antimony speciation in the environment: Recent advances in understanding the biogeochemical processes and ecological effects. J. Environ. Sci. 2019, 75, 14–39. [Google Scholar] [CrossRef]
- Periferakis, A.; Caruntu, A.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, toxicology and medical significance of antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, C.; Gong, D.; Deng, Y.; Huang, Y.; Zheng, J.; Xiong, S.; Tang, R.; Wang, Y.; Su, L. A review of the environmental chemical behavior, detection and treatment of antimony. Environ. Technol. Innov. 2021, 24, 102026. [Google Scholar] [CrossRef]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef]
- Koyuncu, M.; Alwazeer, D. Determination of trace elements, heavy metals, and antimony in polyethylene terephthalate–bottled local raw cow milk of Iğdır region in Turkey. Environ. Monit. Assess. 2019, 191, 666. [Google Scholar] [CrossRef]
- Bolan, N.; Kumar, M.; Singh, E.; Kumar, A.; Singh, L.; Kumar, S.; Keerthanan, S.; Hoang, S.A.; El-Naggar, A.; Vithanage, M.; et al. Antimony contamination and its risk management in complex environmental settings: A review. Environ. Int. 2022, 158, 106908. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- US EPA. National Primary Drinking Water Regulations; US EPA: Washington, DC, USA, 2009. [Google Scholar]
- Masac, J.; Machynak, L.; Lovic, J.; Beinrohr, E.; Cacho, F. On-line electrochemical preconcentration and electrochemical hydride generation for determination of antimony by high-resolution continuum source atomic absorption spectrometry. Talanta 2021, 223, 121767. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.-J.; Hu, X.; Lian, H.-Z. Magnetic solid phase extraction for the determination of trace antimony species in water by inductively coupled plasma mass spectrometry. Talanta 2015, 134, 292–297. [Google Scholar] [CrossRef]
- Dong, L.; Wang, W.; Ning, Y.; Deng, X.; Gao, Y. Detection of trace antimony by vanadium (IV) ion assisted photochemical vapor generation with inductively coupled plasma mass spectrometry measurement. Anal. Chim. Acta 2023, 1251, 341006. [Google Scholar] [CrossRef]
- Hamza, A.; Alsaggaf, W.T.; Ahmad, W.; El-Shahawi, M.S. A highly selective electrochemical sensor for trace determination and speciation of antimony (III & V) in water and soil samples using 2-thenoyltrifluoroacetone. Chem. Pap. 2020, 74, 2917–2927. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Matsuda, Y.; Mori, T.; Uehara, A.; Ishikawa, Y.; Endo, M.; Shida, J. Visual Colorimetry For Trace Antimony(V) by Ion-Pair Solid-Phase Extraction with Bis[2-(5-Chloro-2-Pyridylazo)-5-Diethylaminophenolato]cobalt(III) on a PTFE Type Membrane Filter. Anal. Sci. 2008, 24, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Jardim, W.F.; Dórea, J.G. A simple spectrophotometric procedure for the determination of antimony (III) and (V) in antileishmanial drugs. Fresenius’ J. Anal. Chem. 1997, 358, 548–550. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Wang, L.; Ni, H.; Cao, Z.; Wu, M. A pyrene-based probe for antimony with special excimer fluorescence. Z. Für Anorg. Und Allg. Chem. 2020, 646, 1303–1308. [Google Scholar] [CrossRef]
- Tolessa, T.; Tan, Z.-Q.; Liu, J.-F. Hydride generation coupled with thioglycolic acid coated gold nanoparticles as simple and sensitive headspace colorimetric assay for visual detection of Sb(III). Anal. Chim. Acta 2018, 1004, 67–73. [Google Scholar] [CrossRef]
- Refera, B.; Bekana, D.; Temesgen, A.; Teju, E.; Jabessa, A. Bioinspired gold nanoparticles-based colorimetric nanosensor for detection of antimony in plastic bottled water. Res. Chem. Intermed. 2023, 49, 3227–3244. [Google Scholar] [CrossRef]
- Berlina, A.N.; Komova, N.S.; Zherdev, A.V.; Gaur, M.S.; Dzantiev, B.B. Colorimetric technique for antimony detection based on the use of gold nanoparticles conjugated with poly-A oligonucleotide. Appl. Sci. 2019, 9, 4782. [Google Scholar] [CrossRef]
- Bilge, S.; Karadurmus, L.; Sınağ, A.; Ozkan, S.A. Green synthesis and characterization of carbon-based materials for sensitive detection of heavy metal ions. TrAC Trends Anal. Chem. 2021, 145, 116473. [Google Scholar] [CrossRef]
- Qi, X.; Yan, X.; Zhao, Y.; Li, L.; Wang, S. Highly sensitive and specific detection of small molecules using advanced aptasensors based on split aptamers: A review. TrAC Trends Anal. Chem. 2020, 133, 116069. [Google Scholar] [CrossRef]
- Kumar Kulabhusan, P.; Hussain, B.; Yüce, M. Current Perspectives On Aptamers As Diagnostic Tools And Therapeutic Agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef]
- Shin, W.-R.; Ahn, G.; Lee, J.-P.; Oh, I.-H.; Ahn, J.-Y.; Kim, Y.-H.; Chae, S. Recent Advances in Engineering Aptamer-based Sensing and Recovery of Heavy Metals and Rare Earth Elements for Environmental Sustainability. Chem. Eng. J. 2023, 472, 144742. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Rouhi, N.; Abnous, K.; Taghdisi, S.M.; Verdian, A. Chapter 5—Aptamer-based analysis of heavy metal ions. In Aptamers for Food Applications; Dong, Y., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 127–137. [Google Scholar]
- Li, Y.; Liu, J.-M.; Han, F.; Jiang, Y.; Yan, X.-P. Probing interactions of antimony species with DNA by short column capillary electrophoresis coupled with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2011, 26, 94–99. [Google Scholar] [CrossRef]
- Zhakenovich, A.Y.; Valentina, Y.; Ruben, S.; Spataru, T.; Bykov, A. Synthesis and X-ray study the complexes of ATP with Sb (III) and Bi (III). J. Chem 2016, 10, 195–197. [Google Scholar] [CrossRef]
- Sheng, Y.-M.; Liang, J.; Xie, J. Indirect competitive determination of tetracycline residue in honey using an ultrasensitive gold-nanoparticle-linked aptamer assay. Molecules 2020, 25, 2144. [Google Scholar] [CrossRef]
- Xie, J.; Tang, M.-Q.; Chen, J.; Zhu, Y.-H.; Lei, C.-B.; He, H.-W.; Xu, X.-H. A sandwich ELISA-like detection of C-reactive protein in blood by citicoline-bovine serum albumin conjugate and aptamer-functionalized gold nanoparticles nanozyme. Talanta 2020, 217, 121070. [Google Scholar] [CrossRef]
- Tao, Z.; Wei, L.; Wu, S.; Duan, N.; Li, X.; Wang, Z. A colorimetric aptamer-based method for detection of cadmium using the enhanced peroxidase-like activity of Au–MoS2 nanocomposites. Anal. Biochem. 2020, 608, 113844. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Q.; He, C.; Duan, N. Colorimetric aptasensor for the detection of mercury based on signal intensification by rolling circle amplification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117387. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Y.; Duan, N.; Wang, Z. A colorimetric aptamer sensor based on the enhanced peroxidase activity of functionalized graphene/Fe3O4-AuNPs for detection of lead (II) ions. Catalysts 2020, 10, 600. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, G.; Lin, R.; Pei, X.; Liu, F.; Li, N. Pre-incubation of auric acid with DNA is unnecessary for the formation of DNA-templated gold nanoclusters. Chem. Asian J. 2016, 11, 1677–1681. [Google Scholar] [CrossRef]
- He, W.; Huang, K. Poly-adenine DNA as the high-affinity goalkeeper of mesoporous silica nanoparticles for molecular encapsulation and signal-amplification detection. Surf. Interfaces 2021, 23, 100933. [Google Scholar] [CrossRef]
- Das, A.; Bhadra, K.; Achari, B.; Chakraborty, P.; Kumar, G.S. Interaction of aristololactam-β-D-glucoside and daunomycin with poly(A): Spectroscopic and calorimetric studies. Biophys. Chem. 2011, 155, 10–19. [Google Scholar] [CrossRef]
- Basu, A.; Jaisankar, P.; Kumar, G.S. 9-O-N-aryl/arylalkyl amino carbonyl methyl substituted berberine analogues induce self-structure in polyadenylic acid. RSC Adv. 2012, 2, 7714–7723. [Google Scholar] [CrossRef]
- Vorlíčková, M.; Kejnovská, I.; Bednářová, K.; Renčiuk, D.; Kypr, J. Circular dichroism spectroscopy of DNA: From duplexes to quadruplexes. Chirality 2012, 24, 691–698. [Google Scholar] [CrossRef]
- Santamaría-Díaz, N.; Méndez-Arriaga, J.M.; Salas, J.M.; Galindo, M.A. Highly stable double-stranded DNA containing sequential silver(I)-mediated 7-deazaadenine/thymine Watson–Crick base pairs. Angew. Chem. Int. Ed. 2016, 55, 6170–6174. [Google Scholar] [CrossRef] [PubMed]
- Escher, D.; Müller, J. Silver(I) Coordination in silver(I)-mediated homo base pairs of 6-pyrazolylpurine in DNA duplexes involves the Watson–Crick edge. Chem. A Eur. J. 2020, 26, 16043–16048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Morita, Y.; Sakuragawa, A.; Isozaki, A. Inorganic speciation of As(III, V), Se(IV, VI) and Sb(III, V) in natural water with GF-AAS using solid phase extraction technology. Talanta 2007, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, L.; Zhan, S.; Wang, F.; Zhou, P. Ultrasensitive aptamer biosensor for arsenic(iii) detection in aqueous solution based on surfactant-induced aggregation of gold nanoparticles. Analyst 2012, 137, 4171–4178. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Zhou, P.; Tao, H.; Wang, X.; Wu, Y. Oligonucleotide-induced regulation of the oxidase-mimicking activity of octahedral Mn(3)O(4) nanoparticles for colorimetric detection of heavy metals. Mikrochim. Acta 2020, 187, 99. [Google Scholar] [CrossRef]
- Unutkan, T.; Koyuncu, İ.; Diker, C.; Fırat, M.; Büyükpınar, Ç.; Bakırdere, S. Accurate and sensitive analytical strategy for the determination of antimony: Hydrogen Assisted t-shaped slotted quartz tube-atom trap-flame atomic absorption spectrometry. Bull. Environ. Contam. Toxicol. 2019, 102, 122–127. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, C.; Yu, P.; Zheng, H.; Liu, Z.; Xing, Z.; Hu, S. Determination of antimony in water samples by hydride generation coupled with atmospheric pressure glow discharge atomic emission spectrometry. J. Anal. At. Spectrom. 2019, 34, 331–337. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Regidor-Alfageme, E.; Durazo, A.; Field, J.A.; Umlauf, K.; Sierra-Alvarez, R. LC-ICP-OES method for antimony speciation analysis in liquid samples. J. Environ. Sci. Health Part A 2020, 55, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Panarin, A.Y.; Khodasevich, I.A.; Gladkova, O.L.; Terekhov, S.N. Determination of Antimony By Surface-Enhanced Raman Spectroscopy. Appl. Spectrosc. 2014, 68, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, Z.; Zhang, C.; Song, X.; Song, X.; Zhang, Z. Antimony speciation on a dithiothreitol-functionalized two-dimensional Au@Ag array by surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2022, 373, 132607. [Google Scholar] [CrossRef]
- Tighe, M.; Edwards, M.M.; Cluley, G.; Lisle, L.; Wilson, S.C. Colorimetrically determining total antimony in contaminated waters and screening for antimony speciation. J. Hydrol. 2018, 563, 84–91. [Google Scholar] [CrossRef]
- Moustafa, I.M.I.; Amin, A.S.; Darwish, E. A novel bulk optode for ultra-trace detection of antimony coupled with spectrophotometry in food and environmental samples. Talanta Open 2023, 7, 100197. [Google Scholar] [CrossRef]
- Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Low-tech test for mercury detection: A new option for water quality assessment. Chemosensors 2022, 10, 413. [Google Scholar] [CrossRef]
- Berlina, A.N.; Komova, N.S.; Zherdev, A.V.; Dzantiev, B.B. Combination of phenylboronic acid and oligocytosine for selective and specific detection of lead(ii) by lateral flow test strip. Anal. Chim. Acta 2021, 1155, 338318. [Google Scholar] [CrossRef]







| Samples | Added, ng/mL | Found, ng/mL | Recovery, % |
|---|---|---|---|
| Drinking water | 10.0 | 10.9 ± 0.3 | 109.0 ± 2.8 |
| 50.0 | 63.1 ± 0.2 | 126.2 ± 0.3 | |
| 100 | 115.0 ± 0.2 | 115.0 ± 0.2 | |
| Spring water | 10.0 | 11.2 ± 0.3 | 112.0 ± 2.7 |
| 50.0 | 49.8 ± 0.7 | 99.6 ± 1.4 | |
| 100 | 106.1 ± 1.1 | 106.1 ± 1.0 |
| Method | LOD, ng/mL | Probe | Ref. |
|---|---|---|---|
| Sophisticated Instrumental Methods | |||
| Inductively coupled plasma–optical emission spectrometry | 24.9–32.3 | water, basal culture medium, anaerobic sludge plus basal medium | [44] |
| Ion-assisted photochemical vapor generation with inductively coupled plasma mass spectrometry | 0.0047 | lake and river water | [12] |
| Bulk optode coupled with spectrophotometry using 6-(4-(2,4-dihydroxyphenyl)diazenyl)phenyl)−2-oxo-4-phenyl-1,2-dihydro pyridine-3-carbo-nitrileas a ionophore | 0.85 | tap, domestic, sea, ground, lake water, blood plasma, urine | [48] |
| Surface-enhanced Raman scattering using silvered porous silicon and phenylfluorone | 1 | - | [45] |
| T-shaped slotted quartz tube–atom trap–flame atomic absorption spectrometry | 0.75 | mineral water | [42] |
| Excimer fluorescence using pyrene as a sensing probe | 160 | - | [16] |
| Hydride generation coupled with atmospheric pressure glow discharge atomic emission spectrometry | 0.14 | groundwater | [43] |
| Surface-enhanced Raman spectroscopy using dithiothreitol-functionalized two-dimensional Au@Ag array | 1 | natural water | [46] |
| Low-tech methods | |||
| Colorimetric method based on the development of a yellow potassium iodoantimonite complex | 600 | water samples from mine adits | [47] |
| Colorimetric detection using gold nanoparticles modified with poly-adenine aptamer | 10 | drinking water | [19] |
| Ag+-enhanced microplate apta-enzyme assay using poly-adenine aptamer | 1.9 | drinking and spring water | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Sensitive Silver-Enhanced Microplate Apta-Enzyme Assay of Sb3+ Ions in Drinking and Natural Waters. Molecules 2023, 28, 6973. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28196973
Komova NS, Serebrennikova KV, Berlina AN, Zherdev AV, Dzantiev BB. Sensitive Silver-Enhanced Microplate Apta-Enzyme Assay of Sb3+ Ions in Drinking and Natural Waters. Molecules. 2023; 28(19):6973. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28196973
Chicago/Turabian StyleKomova, Nadezhda S., Kseniya V. Serebrennikova, Anna N. Berlina, Anatoly V. Zherdev, and Boris B. Dzantiev. 2023. "Sensitive Silver-Enhanced Microplate Apta-Enzyme Assay of Sb3+ Ions in Drinking and Natural Waters" Molecules 28, no. 19: 6973. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28196973