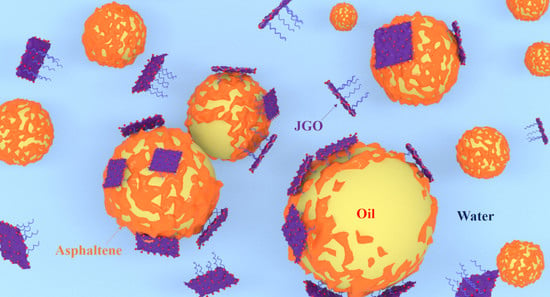
Demulsification of Heavy Oil-in-Water Emulsion by a Novel Janus Graphene Oxide Nanosheet: Experiments and Molecular Dynamic Simulations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of JGO
2.2. Demulsification Efficiency Tests
2.3. Demulsification Mechanism of JGO
2.4. Verification of the Mechanism via Molecular Dynamic Simulation
3. Experimental
3.1. Materials
3.2. Preparation of JGO
3.3. Characterization
3.4. Demulsification Efficiency Test
3.5. Interfacial Dilatational Rheology Measurement
3.6. Simulation Method
3.6.1. Construction of Crude Oil/Water System
3.6.2. Construction of GO and JGO in Crude Oil/Water System
3.6.3. MD Simulation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atta, A.M.; Al-Lohedan, H.A.; Abdullah, M.M.S. Dipoles poly(ionic liquids) based on 2-acrylamido-2-methylpropane sulfonic acid-co-hydroxyethyl methacrylate for demulsification of crude oil water emulsions. J. Mol. Liq. 2016, 222, 680–690. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, J.; Ge, L.; Xu, Z. Demulsifying water-in-oil emulsions by ethyl cellulose demulsifiers studied using focused beam reflectance measurement. Chem. Eng. Sci. 2015, 130, 254–263. [Google Scholar] [CrossRef]
- Kang, W.; Guo, L.; Fan, H.; Meng, L.; Li, Y. Flocculation, coalescence and migration of dispersed phase droplets and oil-water separation in heavy oil emulsion. J. Pet. Sci. Eng. 2012, 81, 177–181. [Google Scholar] [CrossRef]
- Lv, X.; Song, Z.; Yu, J.; Su, Y.; Zhao, X.; Sun, J.; Mao, Y.; Wang, W. Study on the demulsification of refinery oily sludge enhanced by microwave irradiation. Fuel 2020, 279, 118417. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, S.; Zhou, L. Research on the static experiment of super heavy crude oil demulsification and dehydration using ultrasonic wave and audible sound wave at high temperatures. Ultrason. Sonochem. 2018, 40, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Han, J.; Huang, C.; Mu, T. Demulsification of Water-in-Oil Emulsions for the Petroleum Industry by using Alternating Copolymers. Energy Technol. 2014, 2, 618–624. [Google Scholar] [CrossRef]
- Zhao, F.; Tian, Z.; Yu, Z.; Shang, H.; Wu, Y.; Zhang, Y. Research status and analysis of stabilization mechanisms and demulsification methods of heavy oil emulsions. Energy Sci. Eng. 2020, 8, 4158–4177. [Google Scholar]
- Liu, R.; Lu, Y.; Pu, W.; Lian, K.; Sun, L.; Du, D.; Song, Y.; Sheng, J.J. Low-Energy Emulsification of Oil-in-Water Emulsions with Self-Regulating Mobility via a Nanoparticle Surfactant. Ind. Eng. Chem. Res. 2020, 59, 18396–18411. [Google Scholar] [CrossRef]
- Lobo, A.; Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J. Ultrafiltration of oil-in-water emulsions with ceramic membranes: Influence of pH and crossflow velocity. J. Membr. Sci. 2006, 278, 328–334. [Google Scholar] [CrossRef]
- Lv, G.; Gao, F.; Liu, G.; Yuan, S. The properties of asphaltene at the oil-water interface: A molecular dynamics simulation. Colloid Surf. A Physicochem. Eng. Asp. 2017, 515, 34–40. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Xie, L.; Lu, X.; Liu, Q.; He, J.; Mantilla, C.A.; Van den Berg, F.G.A.; Zeng, H. Surface Interaction of Water-in-Oil Emulsion Droplets with Interfacially Active Asphaltenes. Langmuir 2017, 33, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Sjoblom, J.; Wei, D. Interfacial and Emulsion Stabilizing Properties of Indigenous Acidic and Esterified Asphaltenes. J. Dispers. Sci. Technol. 2016, 37, 1751–1759. [Google Scholar] [CrossRef]
- Dai, Q.; Chung, K.H. Hot water extraction process mechanism using model oil sands. Fuel 1996, 75, 220–226. [Google Scholar] [CrossRef]
- Masliyah, J.; Zhou, Z.J.; Xu, Z.H.; Czarnecki, J.; Hamza, H. Understanding water-based bitumen extraction from athabasca oil sands. Can. J. Chem. Eng. 2004, 82, 628–654. [Google Scholar] [CrossRef]
- Sokker, H.H.; El-Sawy, N.M.; Hassan, M.A.; El-Anadouli, B.E. Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization. J. Hazard. Mater. 2011, 190, 359–365. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T.; Ng, K.T.W. Coalescence/Filtration of an Oil-In-Water Emulsion in an Immobilized Mucor rouxii Biomass Bed. Sep. Sci. Technol. 2012, 47, 2241–2249. [Google Scholar]
- Li, H.; Mu, P.; Li, J.; Wang, Q. Inverse desert beetle-like ZIF-8/PAN composite nanofibrous membrane for highly efficient separation of oil-in-water emulsions. J. Mater. Chem. A 2021, 9, 4167–4175. [Google Scholar] [CrossRef]
- Frising, T.; Noik, C.; Dalmazzone, C. The liquid/liquid sedimentation process: From droplet coalescence to technologically enhanced water/oil emulsion gravity separators: A review. J. Dispers. Sci. Technol. 2006, 27, 1035–1057. [Google Scholar] [CrossRef]
- Moosai, R.; Dawe, R.A. Gas attachment of oil droplets for gas flotation for oily wastewater cleanup. Sep. Purif. Technol. 2003, 33, 303–314. [Google Scholar] [CrossRef]
- Fan, C.; Ma, R.; Wang, Y.; Luo, J. Demulsification of Oil-in-Water Emulsions in a Novel Rotating Microchannel. Ind. Eng. Chem. Res. 2020, 59, 8335–8345. [Google Scholar] [CrossRef]
- Dhandhi, Y.; Chaudhari, R.K.; Naiya, T.K. Development in separation of oilfield emulsion toward green technology—A comprehensive review. Sep. Sci. Technol. 2021, 1–27. [Google Scholar] [CrossRef]
- Atta, A.M.; Fadda, A.A.; Abdel-Rahman, A.A.H.; Ismail, H.S.; Fouad, R.R. Application of New Modified Poly(ethylene Oxide)-Block-Poly(propylene oxide)-Block-Poly(ethylene oxide) Copolymers as Demulsifier for Petroleum Crude Oil Emulsion. J. Dispers. Sci. Technol. 2012, 33, 775–785. [Google Scholar] [CrossRef]
- Dalmazzone, C.; Noik, C.; Komunjer, L. Mechanism of crude-oil/water interface destabilization by silicone demulsifiers. Spe J. 2005, 10, 44–53. [Google Scholar] [CrossRef]
- Zhang, L.; Ying, H.; Yan, S.; Zhan, N.; Guo, Y.; Fang, W. Hyperbranched poly(amido amine) demulsifiers with ethylenediamine/1,3-propanediamine as an initiator for oil-in-water emulsions with microdroplets. Fuel 2018, 226, 381–388. [Google Scholar] [CrossRef]
- Hao, L.; Jiang, B.; Zhang, L.; Yang, H.; Sun, Y.; Wang, B.; Yang, N. Efficient Demulsification of Diesel-in-Water Emulsions by Different Structural Dendrimer-Based Demulsifiers. Ind. Eng. Chem. Res. 2016, 55, 1748–1759. [Google Scholar] [CrossRef]
- Jabbari, M.; Izadmanesh, Y.; Ghavidel, H. Synthesis of ionic liquids as novel emulsifier and demulsifiers. J. Mol. Liq. 2019, 293, 111512. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’l-Razi, A.; Abdullah, L.C.; Elnashaie, S.; Pendashteh, A. Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Li, X.; Jia, W.; Zhao, Y.; Ren, S. Recyclable magnetic graphene oxide for rapid and efficient demulsification of crude oil-in-water emulsion. Fuel 2017, 189, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Nikkhah, M.; Tohidian, T.; Rahimpour, M.R.; Jahanmiri, A. Efficient demulsification of water-in-oil emulsion by a novel nano-titania modified chemical demulsifier. Chem. Eng. Res. Des. 2015, 94, 164–172. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Bao, M.; Yang, X.; Wang, Z. Facile Fabrication of Cyclodextrin-Modified Magnetic Particles for Effective Demulsification from Various Types of Emulsions. Environ. Sci. Technol. 2016, 50, 8809–8816. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Jia, W.; Li, Z.; Zhao, Y.; Ren, S. Demulsification of Crude Oil-in-Water Emulsions Driven by Graphene Oxide Nanosheets. Energy Fuels 2015, 29, 4644–4653. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, J.; Xu, H.; Ma, Z.; Jia, W.; Ren, S. Demulsification of heavy oil-in-water emulsions by reduced graphene oxide nanosheets. RSC Adv. 2016, 6, 106297–106307. [Google Scholar] [CrossRef]
- Xu, H.; Jia, W.; Ren, S.; Wang, J.; Yang, S. Stable and efficient demulsifier of functional fluorinated graphene for oil separation from emulsified oily wastewaters. J. Taiwan Inst. Chem. Eng. 2018, 93, 492–499. [Google Scholar] [CrossRef]
- McCoy, T.M.; Turpin, G.; Teo, B.M.; Tabor, R.F. Graphene oxide: A surfactant or particle? Curr. Opin. Colloid Interface Sci. 2019, 39, 98–109. [Google Scholar] [CrossRef]
- Ma, M.; Xu, M.; Liu, S. Surface Chemical Modifications of Graphene Oxide and Interaction Mechanisms at the Nano-Bio Interface. Acta Chim. Sin. 2020, 78, 877–887. [Google Scholar] [CrossRef]
- Wu, H.; Yi, W.; Chen, Z.; Wang, H.; Du, Q. Janus graphene oxide nanosheets prepared via Pickering emulsion template. Carbon 2015, 93, 473–483. [Google Scholar] [CrossRef]
- Contreras Ortiz, S.N.; Cabanzo, R.; Mejía-Ospino, E. Crude oil/water emulsion separation using graphene oxide and amine-modified graphene oxide particles. Fuel 2019, 240, 162–168. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, F.; Zheng, H.; Ren, Z.; Jiang, L.; Ren, Z. Electrostatic-attraction-induced high internal phase emulsion for large-scale synthesis of amphiphilic Janus nanosheets. Chem. Commun. 2019, 55, 1318–1321. [Google Scholar] [CrossRef]
- Herdes, C.; Ervik, Å.; Mejía, A.; Müller, E.A. Prediction of the water/oil interfacial tension from molecular simulations using the coarse-grained SAFT-γ Mie force field. Fluid Phase Equilibria 2018, 476, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.; Mansoori, G.A. The Role of Supercritical/Dense CO2 Gas in Altering Aqueous/Oil Interfacial Properties: A Molecular Dynamics Study. Energy Fuels 2018, 32, 2095–2103. [Google Scholar] [CrossRef]
- Stephan, S.; Hasse, H. Interfacial properties of binary mixtures of simple fluids and their relation to the phase diagram. Phys. Chem. Chem. Phys. 2020, 22, 12544–12564. [Google Scholar] [CrossRef]
- Nagl, R.; Zeiner, T.; Zimmermann, P. Interfacial Mass Transfer in Quaternary Liquid-Liquid Systems. Chem. Eng. Process. Process Intensif. 2022, 171, 108501. [Google Scholar] [CrossRef]
- Stephan, S.; Schaefer, D.; Langenbach, K.; Hasse, H. Mass transfer through vapour–liquid interfaces: A molecular dynamics simulation study. Mol. Phys. 2020, 119, e1810798. [Google Scholar] [CrossRef]
- Baidakov, V.G.; Protsenko, S.P. Molecular-Dynamics Simulation of Relaxation Processes at Liquid–Gas Interfaces in Single- and Two-Component Lennard-Jones Systems. Colloid J. 2019, 81, 491–500. [Google Scholar] [CrossRef]
- Braga, C.; Muscatello, J.; Lau, G.; Muller, E.A.; Jackson, G. Nonequilibrium study of the intrinsic free-energy profile across a liquid-vapour interface. J. Chem Phys. 2016, 144, 044703. [Google Scholar] [CrossRef]
- Stephan, S.; Hasse, H. Enrichment at vapour–liquid interfaces of mixtures: Establishing a link between nanoscopic and macroscopic properties. Int. Rev. Phys. Chem. 2020, 39, 319–349. [Google Scholar] [CrossRef]
- Stephan, S.; Becker, S.; Langenbach, K.; Hasse, H. Vapor-liquid interfacial properties of the system cyclohexane + CO2: Experiments, molecular simulation and density gradient theory. Fluid Phase Equilibria 2020, 518, 112583. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ge, H.; Qiao, L. Molecular Dynamics Simulations of Vapor-Liquid Interface Properties of n-Heptane/Nitrogen at Subcritical and Transcritical Conditions. J. Phys. Chem B 2021, 125, 6968–6985. [Google Scholar] [CrossRef]
- Lian, P.; Jia, H.; Wei, X.; Han, Y.; Wang, Q.; Dai, J.; Wang, D.; Wang, S.; Tian, Z.; Yan, H. Effects of zwitterionic surfactant adsorption on the component distribution in the crude oil droplet: A molecular simulation study. Fuel 2021, 283, 119252. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Tian, S.; Yan, H. Molecular Dynamics Simulation of Emulsification/Demulsification with a Gas Switchable Surfactant. J. Phys. Chem. C 2019, 123, 25246–25254. [Google Scholar] [CrossRef]
- Lucassen-Reynders, E.H.; Lucassen, J. Surface dilational viscosity and energy dissipation. Colloids Surf. A Physicochem. Eng. Asp. 1994, 85, 211–219. [Google Scholar] [CrossRef]
- Falls, A.H.; Scriven, L.E.; Davis, H.T. Adsorption, structure, and stress in binary interfaces. J. Chem. Phys. 1983, 78, 7300–7317. [Google Scholar] [CrossRef]
- Boek, E.S.; Yakovlev, D.S.; Headen, T.F. Quantitative Molecular Representation of Asphaltenes and Molecular Dynamics Simulation of Their Aggregation. Energy Fuels 2009, 23, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Khalaf, M.H.; Mansoori, G.A. A new insight into asphaltenes aggregation onset at molecular level in crude oil (an MD simulation study). J. Pet. Sci. Eng. 2018, 162, 244–250. [Google Scholar] [CrossRef]
- Luo, D.; Wang, F.; Zhu, J.; Cao, F.; Liu, Y.; Li, X.; Willson, R.C.; Yang, Z.; Chu, C.-W.; Ren, Z. Nanofluid of graphene-based amphiphilic Janus nanosheets for tertiary or enhanced oil recovery: High performance at low concentration. Proc. Natl. Acad. Sci. USA 2016, 113, 7711–7716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, M.J.; Tirado-Rives, J.; Jorgensen, W.L. Improved Peptide and Protein Torsional Energetics with the OPLS-AA Force Field. J. Chem. Theory Comput. 2015, 11, 3499–3509. [Google Scholar] [CrossRef]
- Dodda, L.S.; de Vaca, I.C.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.P.; Postma, J.; Gunsteren, W.; Dinola, A.D.; Haak, J.R. Molecular-Dynamics with Coupling to An External Bath. J. Chem. Phys. 1984, 81, 3684. [Google Scholar] [CrossRef] [Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. COMP 8-Canonical sampling through velocity rescaling. Abstr. Pap. Am. Chem. Soc. 2007, 234, 014101. [Google Scholar]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1998, 52, 7182–7190. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Hess, B. P-LINCS: A parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.P.; Ren, S.L. Molecular Dynamics Simulation of Self-Aggregation of Asphaltenes at an Oil/Water Interface: Formation and Destruction of the Asphaltene Protective Film. Energy Fuels 2015, 29, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, Y.; Wang, T.; Zhang, L.; Xu, M.; Jia, H. Demulsification of Heavy Oil-in-Water Emulsion by a Novel Janus Graphene Oxide Nanosheet: Experiments and Molecular Dynamic Simulations. Molecules 2022, 27, 2191. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072191
Xu Y, Wang Y, Wang T, Zhang L, Xu M, Jia H. Demulsification of Heavy Oil-in-Water Emulsion by a Novel Janus Graphene Oxide Nanosheet: Experiments and Molecular Dynamic Simulations. Molecules. 2022; 27(7):2191. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072191
Chicago/Turabian StyleXu, Yingbiao, Yefei Wang, Tingyi Wang, Lingyu Zhang, Mingming Xu, and Han Jia. 2022. "Demulsification of Heavy Oil-in-Water Emulsion by a Novel Janus Graphene Oxide Nanosheet: Experiments and Molecular Dynamic Simulations" Molecules 27, no. 7: 2191. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072191